Chapter 13 Clinical Primary Flexor Tendon Repair and Rehabilitation
A The Bern Experience
Outline
Functional results after flexor tendon repair in zone 2 have markedly improved over the past three decades.1–4 However, a big dilemma of flexor tendon repair in zone 2 remains the challenge between scar formation and risks of rupturing the repairs. Not only repair techniques but also postoperative mobilization require a balance between these two extremes.
The repair that Lim and Tsai advocated5,6 was found to have the mechanical strength required for unrestricted active finger flexion in vitro.5,7 We used the Lim/Tsai repair with an early active tendon motion regimen and/or place-and-hold exercise between 2003 and 2005. We noted that despite the need to mobilize repaired flexor tendons early, an active motion protocol may not be possible to apply in a substantial number of patients due to concomitant injuries, the quality of the surgical repair, or patient factors such as swelling, pain, or lack of compliance. We therefore started a staged rehabilitation program using the “stop and go” principal in the style of a traffic signal: early active controlled flexion (green), place-and-hold (yellow), or passive flexion exercises (red) depending on different factors of the patient such as the severity of injury, the ease of repair or the postoperative compliance.
Methods and Outcomes
Patients
The results were compared to the results of zone 2 flexor tendon repair with the two-strand modified Kessler repair combined with a Kleinert-Duran regimen in 25 patients (30 digits) between 1998 and 2002.1 Both groups included consecutive patients who sustained sharp and complete flexor digitorum profundus (FDP) tendon lacerations in zone 2, with or without concomitant flexor digitorum superficialis (FDS) tendon lacerations or neurovascular damage. Injured tendons in these patients were repaired surgically within 2 days after trauma and postoperative therapies of the patients were continued for a minimum of 8 weeks (Table 13[A]-1).
During the follow-up, grip strength (kg) was measured using a hand-held Jamar Dynamometer (Preston, Jackson, MI). Total active motion (TAM) of each operated finger without secondary surgery was recorded. The flexion of each involved joint was measured first when the patient attempted to make a complete fist; the extension deficits were measured afterward. The original Strickland grading system was used to assess final TAM.8 The functional results were recorded at a mean of 12 weeks postoperatively in both groups (range: Lim/Tsai repair group, 9 to 17 weeks; Kessler repair group, 8 to 16 weeks). The follow-up period was determined on the basis of collected data of the Kessler repair group; the retrospectively collected data of this group provided exact TAM values up to 12 weeks. After 12 weeks, the patient files often contained only qualitative statements without mentioning the degrees of TAM or only giving the remaining extension deficits.
From August 2006 to June 2007, we treated 30 consecutive patients with flexor tendon injury in zone 2 using the Lim/Tsai surgical repair and the postsurgical “stop and go” motion protocol. Exclusion criteria into this review were the same as given earlier1; 26 long finger injuries (involving 25 FDP and 14 FDS tendons) and 9 thumbs were included. The surgeon initially decided on which rehabilitation protocol was used, taking in account the severity of the injury and the quality of the repair.
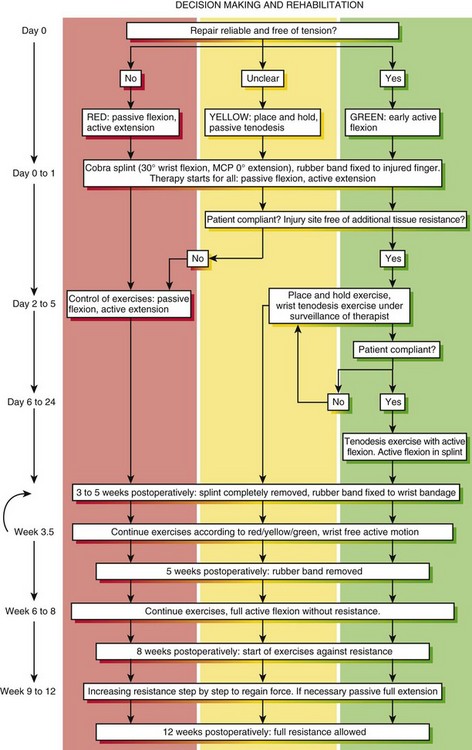
In this most recent cohort of patients, we implemented a new motion protocol—“stop and go” protocol, as detailed in Figure 13(A)-1. At the end of the therapy (postsurgical 3 months ± 1 week) the outcome was measured using the original Strickland classification for the fingers and the Buck-Gramcko classification for the thumbs.
Operative Techniques
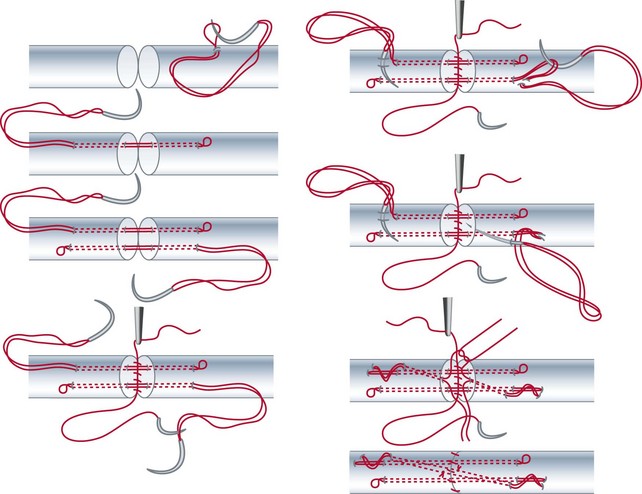
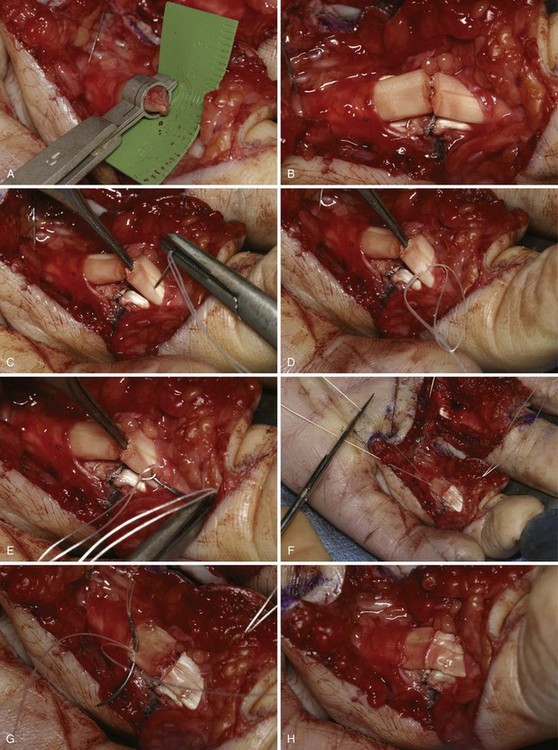
After Bruner zigzag incisions, the core suture was achieved with the six-strand Lim/Tsai5,6 double-loop technique with polyamid (4-0 Supramid; S. Jackson, Alexandria, VA) and an epitendinous running suture with polypropylen (5-0 Prolene) (Figure 13[A]-2). The two slips of the FDS tendon were repaired with the use of simple core Kessler sutures with polyamid single suture (4-0 Supramid). No repair of the tendon sheath was performed.
Postoperative Care
Therapists decide after consulting the surgeon as to which protocols to be used depending on postoperative hand conditions (swelling, pain) and the compliance of the patient. Therapists may change the protocols during the process of rehabilitation (see Figure 13[A]-1).
The Staged Rehabilitation Group: “Stop and Go”
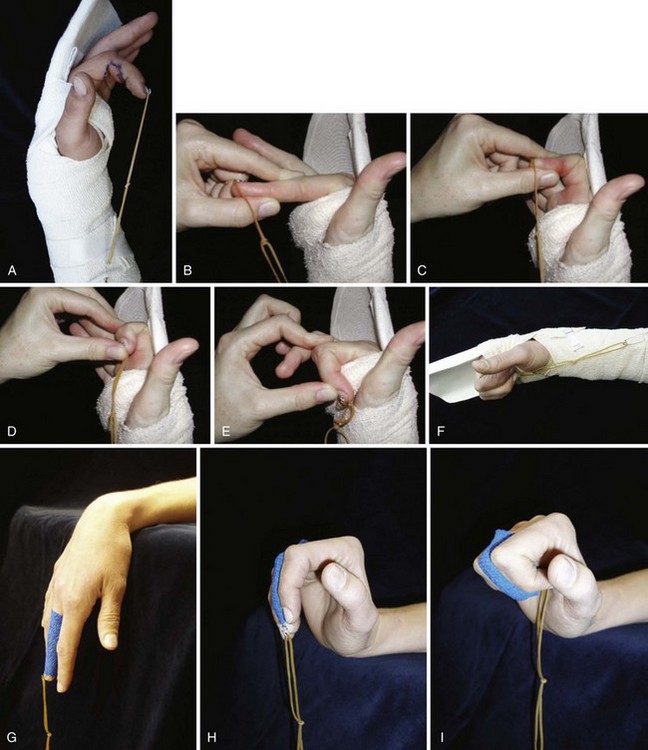
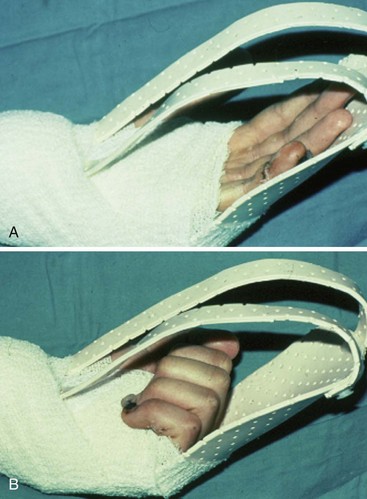
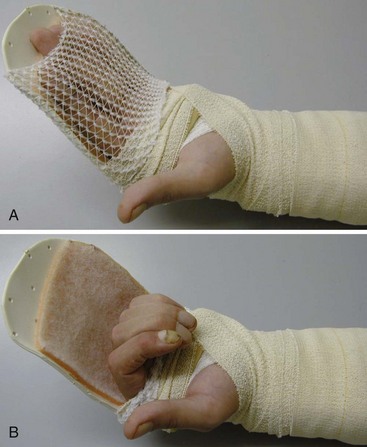
Conditions for the “stop and go” program are that the patient is older than 12 years and that the patient speaks and understands German, French, or English. “Stop and go” is used for lesions in zones 1 to 4; the wrist tenodesis exercises are not performed for zone 5. Immediately after surgery, all patients receive a dorsal “cobra” splint in 30° to 40° wrist flexion, with metaphalangeal (MP) and interphalangeal (IP) joints in 0° (Figure 13[A]-3A).
Red: Passive Flexion, Active Extension
The patient is instructed to perform passive flexion (three times a day) and active extension (hourly) of all fingers. The extension of involved digits should reach the dorsal splint (0° in all finger joints) (Figure 13[A]-3B–E). The goal of passive flexion is to make the involved digits reach the palm.
Yellow: Place and Hold
To the basic program (red), place-and-hold exercises are added. The place-and-hold exercises are done after the basic exercises to decrease tissue resistance. In doing place-and-hold exercises, all fingers are passively flexed and actively hold in the flexed position for one second (Figure 13[A]-3F). During the therapy session, the splint is removed to perform wrist tenodesis exercises to initiate tendon gliding: active extension of the wrist while the fingers stay relaxed (Figure 13[A]-3G–I). After 3.5 weeks, the splint is removed. The rubber band is fixed to a wrist bandage and the exercises are continued in the same way. After 5 weeks, the therapy is continued in the way as described in the red protocol.
Outcomes
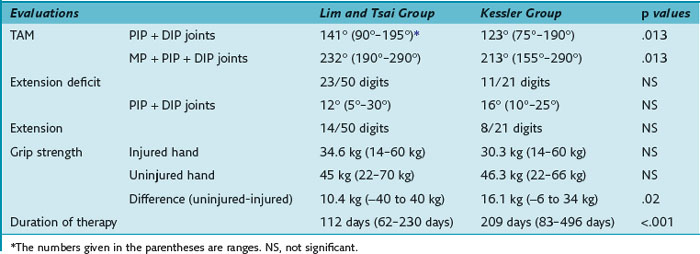
Our results of the two- and six-strand repair are summarized in Table 13(A)-2. The assessment of range of motion, evaluated by the original Strickland classification system, rendered 21 of 50 excellent and 18 of 50 good results in the group of Lim/Tsai repair compared to 4 of 21 and 5 of 21 in the group of Kessler repair. The TAM of the group of Lim/Tsai was 141.4°, which is significantly better (p = 0.013) than the TAM of 123.3° in the Kessler group. In the group of Lim/Tsai, 14 of 50 fingers (12 of 45 patients) (28%) necessitated an extension splint compared to 8 of 21 fingers (8 of 20 patients) (38%) in the group of Kessler. On average, extension splinting was applied around 8.5 weeks postoperatively. The average extension lag was 20.7° in the group of Lim/Tsai and 18.8° in the group of Kessler before splinting. Twelve weeks postsurgery, the remaining extension deficits of the interphalangeal joints were 12° and 16.4°, respectively, demonstrating no statistically significant difference between the two groups. Using the linear model, in the group of Lim/Tsai, the grip strength was significantly better (p = 0.02), and the average time of treatment for long fingers was significantly shorter compared to the group of Kessler (p < 0.0001).
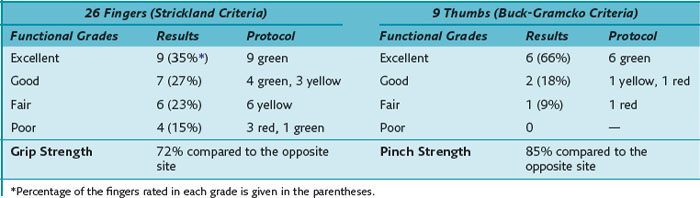
The results of the FPL repair were good or excellent in 90% (6 excellent, 2 good, 1 fair) of digits. The mean active range of motion of the thumb was 61° of flexion and 5° hyperextension in the MP joint and 39° of flexion in the IP joint but with a slight extension lag of 5° (Table 13[A]-3).
Complications
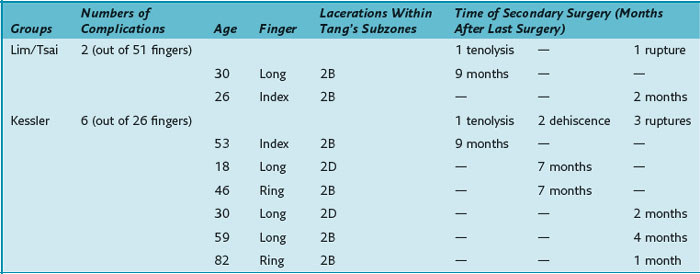
All eight patients requiring secondary surgery had complete lacerations of both the FDP and the FDS tendons. Table 13(A)-4 details the injured finger and the laceration site within zone 2 according to Tang’s subdivision of zone 2.9
Discussion
Gill and colleagues5 conducted a human cadaveric study and showed the superiority of the Lim/Tsai four-strand repair over two- or four-strand repair techniques with regard to tensile strength. Xie and coworkers7 compared different six-strand suture configurations and showed that in vitro the modified Savage and the Tang suture have greater tensile strength than the Lim/Tsai suture, but they also concluded that the Lim/Tsai suture has the mechanical strength for unrestricted active finger flexion. The loop-suture technique simplifies flexor tendon repair compared to the modified Savage repair and does not use superficial knots like the Tang suture. Given the tensile strength of the Lim/Tsai suture, our comparison of the six-strand and two-strand repair techniques1 demonstrated a better outcome without increasing the risk of tendon ruptures using place-and-hold exercises postoperatively in the Lim/Tsai suture group. This group demonstrated a significantly better TAM. There was no increased rate of ruptures, nor did it differ significantly between the two groups. Even without a statistically relevant difference, there may still be a clinical difference given the drop of the rupture rate from 11% to 2% between the Kessler and the Lim/Tsai groups. The rupture rate in group of Lim/Tsai and in the group of Kessler is in line with the published rupture rate of 4% to 10% in zone 2 mentioned in the review of Tang.10 Furthermore, the overall complication rate (including tenolysis and dehiscence) was significantly lower in the group of Lim/Tsai.
Tang and colleagues11 performed a biomechanical study showing that gliding curves of a small diameter and gliding over the rim of a major pulley are associated with an increased rupture risk. Dowd and coworkers14 stated that these characteristics were typically found in zone 2B repairs and repairs of tendons in the little finger (zone 2B defined by Tang9: subzone from the proximal margin of the FDS insertion to the distal border of the A2 pulley). In the clinical study by Dowd and colleagues,12 32 of 42 fingers requiring rerepair had injuries in zone 2B and/or in the little finger. In our study, no repair ruptured in the small finger. Four of six patients with ruptures or dehiscences, had undergone repair in zone 2B (see Table 13[A]-4), which seems to support Dowd and colleagues’ conclusion of zone 2B as the repair site at highest risk. The remaining two patients required repairs in zone 2D (subzone from the proximal border of the A2 pulley to the proximal margin of zone 2).
Using the original Strickland classification, the group of Lim/Tsai demonstrated 39 of 50 (78%) good and excellent results compared to 9 of 21 (43%) in the group of Kessler. Compared to the 50% to 100% good and excellent results of other studies,13–21 the outcome in the group of Kessler repair seemed to be rather poor. The results of the group of Lim/Tsai are in line with the above mentioned reports of zone 2 tendon repairs, especially in view of the longer follow-up of at least at 6 to 12 months in these studies. The only report on Lim/Tsai sutures with place-and-hold exercises in zone 2 is by Lim and Tsai.6 They achieved 81% good/excellent results after 7 months assessed by the revised Strickland criteria compared to 100% of good/excellent results in the group of Lim/Tsai and 91% in the group of Kessler after 3 months in our series when using the revised Strickland criteria.
1 Hoffmann GL, Büchler U, Vögelin E. Clinical results of flexor tendon repair in zone II using a six-strand double-loop technique compared with a two-strand technique. J Hand Surg (Eur). 33, 2008. 418–123
2 Strickland JW. The scientific basis for advances in flexor tendon surgery. J Hand Ther. 2005;18:94-110.
3 Al-Qattan MM, Al-Turaiki TM. Flexor tendon repair in zone 2 using a six-strand ‘figure of eight’ suture. J Hand Surg (Eur). 2009;34:322-328.
4 Trumble TE, Vedder NB, Seiler JGIII, et al. Zone-II flexor tendon repair: A randomized prospective trial of active place-and-hold therapy compared with passive motion therapy. J Bone Joint Surg (Am). 2010;92:1381-1389.
5 Gill RS, Lim BH, Shatford RA, et al. A comparative analysis of the six-strand double-loop flexor tendon repair and three other techniques: A human cadaveric study. J Hand Surg (Am). 1999;24:1315-1322.
6 Lim BH, Tsai TM. The six-strand technique for flexor tendon repair. Atlas Hand Clin. 1996;1:65-76.
7 Xie RG, Zhang S, Tang JB, et al. Biomechanical studies of 3 different six-strand flexor tendon repair techniques. J Hand Surg (Am). 2002;27:621-627.
8 Strickland JW, Glogovac SV. Digital function following flexor tendon repair in Zone II: A comparison of immobilization and controlled passive motion techniques. J Hand Surg (Am). 1980;5:537-543.
9 Tang JB. Flexor tendon repair in zone 2C. J Hand Surg (Br). 1994;19:72-75.
10 Tang JB. Clinical outcomes associated with flexor tendon repair. Hand Clin. 2005;21:199-210.
11 Tang JB, Xu Y, Wang B. Repair of strength of tendons of varying gliding curvature: A study in a curvilinear model. J Hand Surg (Am). 2003;28:243-249.
12 Dowd MB, Figus A, Harris SB, et al. The results of immediate re-repair of zone 1 and 2 primary flexor tendon repairs which rupture. J Hand Surg (Br). 2006;31:507-513.
13 Bainbridge LC, Robertson C, Gillies D, et al. A comparison of post-operative mobilization of flexor tendon repairs with “passive flexion-active extension” and “controlled active motion” techniques. J Hand Surg (Br). 1994;19:517-521.
14 Baktir A, Turk CY, Kabak S, et al. Flexor tendon repair in zone 2 followed by early active mobilization. J Hand Surg (Br). 1996;21:624-628.
15 Cullen KW, Tolhurst P, Lang D, et al. Flexor tendon repair in zone 2 followed by controlled active mobilisation. J Hand Surg (Br). 1989;14:392-395.
16 Elliot D, Moiemen NS, Flemming AFS, et al. The rupture rate of acute flexor tendon repairs mobilized by the controlled active motion regimen. J Hand Surg (Br). 1994;19:607-612.
17 Klein L. Early active motion flexor tendon protocol using one splint. J Hand Ther. 2003;16:199-206.
18 Lee H. Double loop locking suture: a technique of tendon repair for early active mobilization. Part II: Clinical experience. J Hand Surg (Am). 1990;15:953-958.
19 May EJ, Silfverskiold KL, Sollerman CJ. Controlled mobilization after flexor tendon repair in zone II: A prospective comparison of three methods. J Hand Surg (Am). 1992;17:942-952.
20 Silfverskiold KL, May EJ. Flexor tendon repair in zone II with a new suture technique and an early mobilization program combining passive and active flexion. J Hand Surg (Am). 1994;19:53-60.
21 Small JO, Brennen MD, Colville J. Early active mobilisation following flexor tendon repair in zone 2. J Hand Surg (Am). 1989;14:383-391.
B The Chelmsford Experience
Outline
History
Although Bunnell (1918) wrote on primary repair and early, albeit guarded and intermittent, active mobilization, his subsequent experience in the 1920s presumably led to the dictate that flexor tendons divided in the tendon sheath of the fingers should not be repaired primarily but treated by delayed tendon grafting. This practice dominated the practice of flexor tendon surgery for 40 years. The past 50 years is notable for the reversal of this policy and recognition that results after primary or delayed primary flexor tendon repair, that is within a few days of tendon division, can be better than after delayed tendon grafting, provided early surgery is combined with early mobilization of the repaired tendons, a change largely pioneered by Verdan,1 Young and Harman,2 and Kleinert and colleagues.3 Although there is ongoing debate about the details of technique, the central tenet of modern flexor tendon surgery is to repair and move the flexor tendons within a few days of injury. While all flexor tendon surgery is complicated, it is simplest in the newly injured and unscarred digit and the results of correctly rehabilitated primary repairs are likely to be the best attainable.
The Problems of Primary Flexor Tendon Repair
Repair of the divided flexor tendon to achieve normal, or near normal, function is a problem that has not yet been solved. Primary flexor tendon surgery remains technically difficult, with each result still being uncertain. There have been about 20 methods of assessing flexor tendon results described in the hand literature since 1950.4 This repeating need to reevaluate our methods of assessment has come not from attempts to keep up with improvement of the results over the years but from a repeating need to quantify how much we should reasonably downgrade our expectations to accommodate for the imperfections of our treatment. Over and above the actual technical difficulties of repairing tendons, we face the complications of rupture and adherence of repairs during healing and these two problems have dominated thought on this subject for a century. “Spot-welding” by scar adhesions can occur anywhere along the length of a flexor tendon, but it is a particular problem in the fingers themselves, where the flexors are confined within the tendon sheath in a system as finely bored as the pistons in an engine. This requires that the tendons be mobilized throughout the period immediately after repair when tendon continuity depends almost entirely on the strength of the sutures, as healing of the flexor tendon takes about 3 months. Unfortunately, this period is sometimes longer than that for which the hand can be kept free of activities, or accidents, liable to snap the repair. In this unit, our main research interests have been to eliminate rupture of the tendons while maintaining a policy of enthusiastic early active mobilization. The assumption supporting this philosophy is that the results will be better with increasing early movement through the first 5 weeks, albeit within the protective environment of a dorsal splint, provided the sutures hold and rupture does not occur. Our research over 20 years has aimed at liberating rehabilitation from unnecessary constraints while, coincidently, reducing the rupture rate after primary repair.
Early Active Mobilization
The author’s interest in early active mobilization comes from his training with McGrouther in Glasgow, then with Brown and Black in Newcastle, prior to arrival in the Chelmsford unit. The latter part of my training coincided with the publishing of an article by the hand surgeons in Belfast, not far away, in which they described mobilization after routine zone 2 flexor tendon repairs in a Kleinert traction splinting system but without the elastic bands (i.e., actively moving the fingers when flexing as well as when extending).5 This was not actually new as many before had either never used rubber bands or tried to get rid of them,6–25 although always stressing the use of some variant of suture technique to make the repair stronger, presuming this would be necessary to withstand early active movement. What the Belfast surgeons identified was the fact that the sutures did not need to be stronger to allow early active mobilization in both directions. The desire to be free of the rubber bands had been prevalent for years, largely because of the problems arising from the flexed resting position of the proximal interphalangeal joints in Kleinert traction and, also, because of the difficulties in managing Kleinert traction. It was also realized that many patients never actually used the rubber bands to passively flex but simply flexed their fingers actively, even when the bands were correctly tensioned, which was often only for 5 minutes after leaving the therapy department. This stimulated me to repeat the Belfast experiment. This is recorded in an article26 comparing patients mobilized in a passive flexion-active extension, or “Kleinert” regimen,27 with patients mobilized in an active flexion-active extension, or “Belfast” regimen,5 after the same repair with a two-strand modified Kessler core suture and a simple running circumferential suture. This study, performed between 1986 and late 1987 (although only published much later), and the study from Sheffield, UK, reporting the results of their repeat of the Belfast experiment28 convinced me that this was the way forward for rehabilitation, in respect of simplifying rehabilitation to a level commensurate with the availability of therapy in our own unit and likely to be available worldwide. The results of these, and subsequent reports throughout the 1990s, confirmed a rupture rate of around 5% when using variants of the Belfast regimen,5,26–30 which was similar to that reported at the time by units worldwide using the Kleinert regimen.26,29,31–35
Passive Mobilization
The other alternative to the Kleinert technique of mobilization, introduced in the United States by Duran and Houser (1975) and supported by Strickland and Glogovac (1980), in which the fingers were only mobilized passively by a therapist, or the patient’s other hand, was never popular in the United Kingdom as it was very labor (therapy) intensive, with no seeming advantages.36,37 A common debate at the time which, to my knowledge, has never been settled, was whether the tendons actually moved significantly with this regimen or simply bunched up as the fingers were passively flexed. Another factor that made it unattractive was the fact that the first report included a 14% rupture rate,36 while the second had only 56% good and excellent results,37 both of which are unacceptable compared with published results at the time using the Kleinert regimen and, subsequently, the Belfast regimen. The most common use now of the Duran Houser idea is in helping Kleinert and Belfast regimens push for better results at the extremes of movement.
St. Andrew’s Early Results—fingers
Our own early results using a two-strand Tajima modification of the Kirchmayer, or Kessler, core suture19,38 of 3-0 or 4-0 polypropylene (Prolene), in which the suture is tied with a single intratendinous knot, a simple continuous running circumferential suture of 5-0 or 6-0 nylon (Ethilon), or polypropylene, and a modification of the Belfast regimen (Figure 13[B]-1), were reported in 1994.28 Over a period of 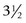 years, 233 patients were treated for complete divisions of flexor tendons in zones 1 and 2 within 24 hours of admission following emergency referral. The patients included 58 complete tendon divisions in 58 fingers in 54 patients in zone 1 and 259 tendon divisions in 166 fingers in 149 fingers in zone 2. A later study extended this survey to include 508 patients with 840 acute complete flexor tendon divisions in 605 fingers treated in the same manner between June 1989 and December 1996.29 These reports were both focused on the problem of rupture. In the first report, an overall rupture rate of 5.8% was achieved, with a rupture rate for zone 2 injuries of 5.4% and for zone 1 injuries of 6.8%, confirming our belief that the regimen was safe for use as an alternative to Kleinert-type regimens for mobilization of zone 1 and 2 finger flexor tendon injuries. In the larger survey, after exclusion of 68 patients who did not complete the 8-week rehabilitation program, 440 patients with 728 complete tendon divisions in 526 fingers were assessed. Twenty-three patients suffered rupture of 28 tendons in 23 fingers, an overall rupture rate of 4%. One hundred and twenty-nine fingers with zone 1 injuries had a rupture rate of 5% and 397 fingers with zone 2 injuries had a rupture rate of 4%. The good and excellent results in the final year of the first study, assessed using the first Strickland method of assessment,37 of 62.5% for zone 1 injuries and 79.4% for zone 2 injuries confirmed that these rates of rupture were not being achieved at the expense of increased protection and poor mobility. These studies confirmed that early active motion was as safe as any current regimen, with results comparable to those of previous studies in terms of both rupture and mobility in a considerable number of fingers, most other studies in the literature being much smaller in size. At the time, we wrote that we believed the Belfast type of rehabilitation was both simpler and less expensive to maintain than the Kleinert type of rehabilitation regimen. We were no longer concerned about the safety of the Belfast regimen, something, which still persists elsewhere, particularly in the United States. However, we had also reached the conclusion that argument over which was the best of the two active regimens was probably unproductive.
years, 233 patients were treated for complete divisions of flexor tendons in zones 1 and 2 within 24 hours of admission following emergency referral. The patients included 58 complete tendon divisions in 58 fingers in 54 patients in zone 1 and 259 tendon divisions in 166 fingers in 149 fingers in zone 2. A later study extended this survey to include 508 patients with 840 acute complete flexor tendon divisions in 605 fingers treated in the same manner between June 1989 and December 1996.29 These reports were both focused on the problem of rupture. In the first report, an overall rupture rate of 5.8% was achieved, with a rupture rate for zone 2 injuries of 5.4% and for zone 1 injuries of 6.8%, confirming our belief that the regimen was safe for use as an alternative to Kleinert-type regimens for mobilization of zone 1 and 2 finger flexor tendon injuries. In the larger survey, after exclusion of 68 patients who did not complete the 8-week rehabilitation program, 440 patients with 728 complete tendon divisions in 526 fingers were assessed. Twenty-three patients suffered rupture of 28 tendons in 23 fingers, an overall rupture rate of 4%. One hundred and twenty-nine fingers with zone 1 injuries had a rupture rate of 5% and 397 fingers with zone 2 injuries had a rupture rate of 4%. The good and excellent results in the final year of the first study, assessed using the first Strickland method of assessment,37 of 62.5% for zone 1 injuries and 79.4% for zone 2 injuries confirmed that these rates of rupture were not being achieved at the expense of increased protection and poor mobility. These studies confirmed that early active motion was as safe as any current regimen, with results comparable to those of previous studies in terms of both rupture and mobility in a considerable number of fingers, most other studies in the literature being much smaller in size. At the time, we wrote that we believed the Belfast type of rehabilitation was both simpler and less expensive to maintain than the Kleinert type of rehabilitation regimen. We were no longer concerned about the safety of the Belfast regimen, something, which still persists elsewhere, particularly in the United States. However, we had also reached the conclusion that argument over which was the best of the two active regimens was probably unproductive.
The 5% Rupture Rate
Throughout this period, we were concerned by the seemingly static state of acute flexor tendon repairs being reported worldwide in those units publishing results. With few exceptions, most studies discussing acute flexor tendon repair and early mobilization reported a rupture rate of between 3% and 6%. In studies of less than 100 patients, these figures indicated negligible differences in the number of patients with ruptured tendon repairs in different units in different countries and continents, using a multitude of suture techniques and variations of the basic regimens of early postoperative mobilization. Even taking into account the variations in methods of assessment used at the time,4,39–41 the similarity of the results in different studies showed that an adequately trained, but not necessarily experienced, surgeon, using a routine repair of divided flexor tendons, whether using the Kirchmayr/Kessler core suture or following the technique described by Tsuge,42,43 and with good therapists, would achieve 70% to 80% excellent, or good, results and suffer a rupture rate of about 5%. The only identifiable way of improving results in the early 1990s, although having little effect on rupture rate, seemed to be to increase postoperative supervision, generally believed to be the explanation of the excellent results achieved by Chow and his colleagues, dealing with military personnel.32 It was thought at the time that this was unattainable in civilian practice, until the civilian surgeons and therapists in Göteborg, in Sweden, showed that—with a stronger technique of circumferential suturing and a more rigorous therapy regimen, incorporating features of the Kleinert, the Duran-Houser, and the Belfast regimens—this was not true.44 However, this study still included two ruptures in 55 repairs: stronger sutures and more complex rehabilitation still had not solved the problem of rupture of primary repairs.
Etiology of Ruptures
Most ruptures in our study occurred with the splint in place. These tendon repairs might have been protected from rupture by better mechanical obstruction of the palm. Rubber bands across the palm have a definite obstructing action, which was not present in the original technique of early active motion.5 However, all of our patients at that time wore a modified Belfast splint, which included wide thermoplastic bars across the open side of the splint, running from the distal edge back to the volar aspect of the wrist and known locally as “beer-can bars” (see Figure 13[B]-1).30 These provided a similar obstruction to grasping to that of the rubber bands of the Kleinert splint. Attempts to increase this feature of splinting and/or attempts to make splints impossible to remove might interfere with rehabilitation and would probably still fail in a proportion of patients. They would certainly have little effect in those who remove the splint to use the hand for grasping. In the last five years, the ‘beer-can bars’ on our splints have given way to a sheath of lightly elasticated material which holds the fingers in extension against the dorsal splint for most of the time, with the sheath being rolled down into the palm for exercising of the fingers (Figure 13[B]-2).
The Splinted Wrist Position
While accepting the work of Savage that the best position for the wrist in respect of minimum firing of both the flexor and extensor muscles is in slight extension,45 we have not had time to research this fully and have only gone halfway from the original flexed position to a straight wrist splint. The actual therapy regimen has not changed since that described in 1994 (Box 13[B]-1).30 The time in the splint remains 5 weeks, followed by 3 weeks weaning from the splint. Light activities start at 8 weeks, with heavy lifting only after 12 weeks. This 5-week period of total protection seems to be in accord with our findings in respect of the timing of rupture of repairs.4,28,46
Rupture Re-Repair
Our interest in the rupture of repairs was taken further in a more recent review of all ruptures of zone 1 and 2 primary flexor tendon repairs performed in our unit between 1989 and 2003.46 Although concern about tendon rupture had been one of the major determinants in the evolution of the various techniques of tendon suture and early postoperative mobilization throughout the past 10 years of the twentieth century, there was still almost no information in the literature about the etiology of tendon rupture.28,47 The intention of our 2006 report46 was to analyze in further detail (1) the reasons for tendon rupture during the early mobilization period and (2) the outcome of immediate re-repair, with a view to identifying whether this should be an invariable policy, whenever possible. Discussion of our views on the management of ruptures of primary repairs of flexor tendons in zones 1 and 2 in the fingers are considered further in Chapter 19.
Venting the Pulleys
In retrospect, a factor in achieving these results that received no attention at the time but was, possibly, of significance was that, from the earliest of the studies in which I was involved, it had been routine to “vent” pulleys as necessary to allow repairs to travel through a full range of excursion on passive movement of the finger after repair without impinging on the A2 or A4 pulleys. The conviction that this “made sense” followed a private conversation as a trainee in the mid-1980s at the Derby Hand Course with Dr. Strickland. At the time, Lister and others were quite adamant that these pulleys should remain entirely intact. Dr. Strickland seemed less certain and I, knowing the problems I personally experienced in the emergency theater, was certain that venting was correct and necessary in many cases. The discussion of “venting” is taken further and, I believe, to its logical conclusion in other chapters of this book and in two review articles in the recent past.48,49 Analyzing the sites along the tendon sheath were tendon injury commonly occurs, Dr. Tang has described appropriate pulley releases for each injury and accords this process of pulley “venting” equal importance as the use of stronger repairs in increasing the margin of safety of early active mobilization.49 We believe the results of zone 1 primary flexor tendon surgery are equally dependent on judicious venting of the A4 pulley.50
Stronger Repairs
At that time, increasing the strength of our sutures seemed likely to be the most effective preventative of tendon rupture in this group of patients. The ability of tendon sutures to withstand the forces of early movement is, probably, the most fundamental limitation to what can be achieved by early movement of primary flexor tendon repairs by any technique. This has been the second prong in the mechanical way forward since Harmer, in Boston, in 1917, introduced a flexor tendon core suture that he believed was sufficiently strong to resist rupture and commenced immediate free movement of the fingers postoperatively without any splints,14 and, in the same year, Kirchmayr presented his stronger suture, with the same purpose in mind.38 Our work on ruptures had convinced us, like others, that we needed stronger sutures to continue aggressive early active mobilization without this 5% rupture rate.
Zone 2 as a Black Box
Throughout the 1990s, we were not entirely happy with the zone 2 model for investigation of the problem further, although zone 2 had become the accepted testing ground for mobilization techniques—and for individual units! It was obvious that most studies reported less than 50 cases and that it was difficult to collect sufficient cases of zone 2 injuries alone in a single unit to make a series. As a result, three things had happened. The first was that the total number of studies remained small. The second was that most studies were small in themselves and, in many cases, too small to be of scientific value. The third was that the available numbers were too small to analyze exactly what was going on in the “black box” we called zone 2, as all the results had to be put together to achieve a publishable total. Zone 2 is far from homogeneous: there are eight permutations of tendon injury in what we call a zone 2 injury. We ignore the three partial injuries and put all those with at least one complete tendon division into the black box marked “zone 2 injury”: there are actually five different injuries in this box. In the 1990s, we looked at this briefly and it looked as if there might be differences in results for different injuries, with a complete flexor digitorum profundus (FDP) and partial FDS division faring worse with our current mobilization than any other combination of tendon lacerations. Another fact that we ignore is that fingers with both tendons cut with the finger flexed have two repair lines that are a long way apart when the finger is extended and only come together in flexion. By contrast, when the finger is cut in extension, the tendon repairs remain together all the time throughout flexion and extension. It might reasonably be expected that the results would differ between these two injuries. A third fact about zone 2 that we ignore in putting all the results together is that the sheath is not a cylinder of unchanging topography along the length of the zone. This is equally true of zone 1.50 So, the tendon environment is varying quite dramatically along both zones. Unfortunately, the circumstances in respect of analysis of zone 2 injuries have not changed. The fact that it is difficult to collect even 50 acute zone 2 finger flexor injuries in a single unit makes for an ongoing problem for those researching new suture configurations in this field: if only 2 or 3 in every 50 repairs rupture, then it takes a long time before we can be sure that any innovation has achieved anything.
The Flexor Pollicis Longus Model
The flexor pollicis longus (FPL) tendon had been researched very little over the previous 30 years, but the extensive literature of an earlier era (1937 to 1960) clearly identified a much higher rupture rate after primary repair than that of the finger flexors.51,52 Surgeons at that time recognized this and debated whether to repair this tendon by insertion of a tendon graft or by lengthening the proximal tendon.52 When we reported our results of zone 1 and 2 finger flexors in 1994,30 we also looked at the FPL results and found a rather horrific rupture rate of 17% in 30 thumbs when mobilized in the Belfast regimen of active flexion/active extension of the repairs. At that time, we recommended that this technique of mobilization not be used in its present form after repair of the long thumb flexor. However, we realized that the higher rupture rate might make the FPL a good clinical model to test new sutures and suture techniques. Using this model, we were able to examine some of the new suture configurations being described in laboratory experiments in a series of studies,52–54 which are described in Chapter 16. Although these reports elaborate an increasingly safer technique for dealing with division of the FPL tendon, they were undertaken largely to examine possible ways forward in respect of the finger flexors. Ultimately, these clinical experiments with the divided FPL achieved zero rupture rates using two different suture techniques (a combination of a four-strand core suture55 and a Silfverskiöld circumferential suture56) and Tang’s three Tsuge suture repair.57,58
To us, this confirmed the likelihood that increase in suture complexity would successfully reduce the rupture rate during early mobilization after repair of the finger flexor tendons and, like most others, we have gone the way of increasing suture complexity. After a dalliance with more complex circumferential sutures, we came to the conclusion that these were too complicated for trainees, who are likely to operate on these cases both in our own unit and worldwide. The combination of a four-strand core suture and a complex circumferential sutures also gave rise to concern about the bulk of the repairs and possible problems of resistance to movement of the repaired tendon.59 This was suggested by a slight drop in the excellent and good results in this group of FPL repairs compared with previous results. However, this problem was small. Of more significance was the fact that these complex sutures are more difficult to use in clinical practice. In our FPL studies, this led to the use of Tang’s triple Tsuge suture technique57,58 for FPL repair, as this is a less complicated suture technique.
Stronger Sutures—dilemmas
Four-strand repairs have become the order of the day for zone 1 and 2 finger flexor repairs at present in Chelmsford. However, there remain two causes for concern as we all move to more complex suturing. Reexamination of the clinical series reported over the past 25 years identifies the study in 1989 by Savage and Risitano,25 which introduced Savage’s six-strand suture and, with it, the search for the ideal multistrand suture that would have the strength of the Savage suture but be simpler to put into the tendon, as the series with the lowest rupture rate of one rupture in 31 zone 2 fingers and 2 thumbs (3%). The next lowest rupture rate was in our series from Chelmsford in 1999, which reported 17 ruptures after zone 1 and 2 primary flexor tendon repairs in 397 fingers (4%).31 This much larger series of cases were repaired with only two-strand Kirchmayr/Kessler core sutures and simple running circumferential suturing, making the hard evidence for improvement of clinical results by more complex core suturing questionable. A very interesting laboratory study from Manchester may identify a further, previously unknown, factor that is concerning.60 It showed that even a single suture passed through a tendon significantly affected the tenocyte cell population of the tendon around it. The suture foreign body caused the tenocytes to move away. So, perhaps, we are unwittingly making tendon repair breakdown more likely as we put in more sutures!
Conclusion
However, given that 10 of every 100 patients undergoing surgery in a good unit will still either experience rupture of the repair or require a tenolysis to free stuck tendons, we should not be satisfied with current practice. While we are still obliged to call results “excellent” when they are only 85% of normal,37 the search for better treatment should continue!
1 Verdan CE. Primary repair of flexor tendons. J Bone Joint Surg (Am). 1960;42:647-657.
2 Young RE, Harmon JM. Repair of tendon injuries of the hand. Ann Surg. 1960;151:562-566.
3 Kleinert HE, Kutz JE, Ashbell T, et al. Primary repair of lacerated flexor tendons in “no man’s land,”. J Bone Joint Surg (Am). 1967;49A:577.
4 Elliot D, Harris SB. The assessment of flexor tendon function after primary tendon repair. Hand Clin. 2003;19:495-503.
5 Small JO, Brennen MD, Colville J. Early active mobilisation following flexor tendon repair in zone 2. J Hand Surg (Br). 1989;14:383-391.
6 Becker H, Orak F, Duponselle E. Early active motion following a bevelled technique of flexor tendon repair: Report on fifty cases. J Hand Surg (Am). 1979;4:454-460.
7 Brunelli G, Vigasio A, Brunelli F. Slip-knot flexor tendon suture in zone 2 allowing immediate mobilisation. Hand. 1983;15:352-358.
8 Bunnell S. Repair of tendons in the fingers and description of two new instruments. Surg Gynecol Obstet. 1918;126:103-110.
9 Emery FE. Immediate mobilization following flexor tendon repair. A preliminary report. J Trauma. 1977;17:1-7.
10 Furlow LT. The role of tendon tissues in tendon healing. Plast Reconstr Surg. 1976;57:39-49.
11 Garlock JH. The repair processes in wounds of tendons, and in tendon grafts. Ann Surg. 1927;85:92-103.
12 Hernandez A, Velasco F, Rivas A, et al. Preliminary report on early mobilization for the rehabilitation of flexor tendons. Plast Reconstr Surg. 1967;40:354-358.
13 Hester TR, Hill L, Nahai F. Early mobilization of repaired flexor tendons within digital sheath using an internal profundus splint: Experimental and clinical data. Ann Plast Surg. 1984;12:187-198.
14 Harmer TW. Tendon suture. Boston Med Surg J. 1917;177:808-810.
15 Harmer TW. Cases of tendon and nerve injury. Boston Med Surg J. 1926;194:739-747.
16 Harmer TW. Injuries to the hand. Am J Surg. 1938;42:638-658.
17 Lahey FH. A tendon suture which permits immediate motion. Boston Med Surg J. 1923;188:851-852.
18 Lexer E. Die Verwerthung der freien Sehnentransplantation. Archiv Klin Chir. 1912;98:818-825.
19 Kessler I, Nissim F. Primary repair without immobilisation of flexor tendon division within the digital sheath. Acta Orthop Scand. 1969;40:587-601.
20 Mangus DJ, Brown F, Byrnes W, et al. Tendon repairs with nylon and a modified pullout technique. Plast Reconstr Surg. 1971;48:32-35.
21 Mantero R, Bertolotti P, Badoini C. II pull-out in “no man’s land” e al canale digitale nelle lesioni dei flessori (metodo personale). Riv Chir Mano. 1973/74;11:119-130.
22 Murray G. A method of tendon repair. Am J Surg. 1960;99:334-335.
23 Nigst H. Chirurgie der Beugesehnen. Handchir. 1976;8:225-236.
24 Pribaz JJ, Morrison WA, Macleod AM. Primary repair of flexor tendons in no-man’s land using the Becker repair. J Hand Surg (Br). 1989;14:400-405.
25 Savage R, Risitano G. Flexor tendon repair using a “six strand” method of repair and early active mobilisation. J Hand Surg (Br). 1989;14:396-399.
26 Bainbridge LC, Robertson C, Gillies D, et al. A comparison of post-operative mobilization of flexor tendon repairs with “passive flexion-active extension” and “controlled active motion” techniques. J Hand Surg (Br). 1994;19:517-521.
27 Cullen KW, Tolhurst P, Lang D, et al. Flexor tendon repair in zone 2 followed by controlled active mobilisation. J Hand Surg (Br). 1989;14:392-395.
28 Elliot D, Moiemen NS, Flemming AFS, et al. The rupture rate of acute flexor tendon repairs mobilized by the controlled active motion regimen. J Hand Surg (Br). 1994;19:607-612.
29 Harris SB, Harris D, Foster AJ, et al. The aetiology of acute rupture of flexor tendon repairs in zones 1 and 2 of the fingers during early mobilization. J Hand Surg (Br). 1999;24:275-280.
30 Baktir A, Türk CY, Kabak S, et al. Flexor tendon repair in zone 2 followed by early active mobilization. J Hand Surg (Br). 1996;21:624-628.
31 Lister GD, Kleinert HE, Kutz JE, et al. Primary flexor tendon repair followed by immediate controlled mobilisation. J Hand Surg (Am). 1977;2:441-451.
32 Chow JA, Thomes LJ, Dovelle S, et al. A combined regimen of controlled motion following flexor tendon repair in “no man’s land,. Plast Reconstr Surg. 1987;79:447-455.
33 Chow JA, Thomes LJ, Dovelle S, et al. Controlled motion rehabilitation after flexor tendon repair and grafting. A multi-centre study. J Bone Joint Surg (Br). 1988;70:591-595.
34 Gault DT. A review of repaired flexor tendons. J Hand Surg (Br). 1987;12:321-325.
35 Saldana MJ, Chow JA, Gerbino P2nd, et al. Further experience in rehabilitation of zone II flexor tendon repair with dynamic traction splinting. Plast Reconstr Surg. 1991;87:543-546.
36 Duran RJ, Houser RG. Controlled passive motion following flexor tendon repairs in zones II and III. In: Hunter JM, Schneider LH, editors. AAOS Symposium on Flexor Tendon Surgery in the Hand. St Louis: CV Mosby; 1975:105-114.
37 Strickland JW, Glogovac SV. Digital function following flexor tendon repair in zone II: A comparison of immobilization and controlled passive motion techniques. J Hand Surg (Am). 1980;5:537-543.
38 Kirchmayr L. Zur Technik der Sehnennaht. Zentralbl Chir. 1917;40:906-907.
39 Amadio PC. Outcome assessment in hand surgery and hand therapy: an update. J Hand Ther. 2001;14:63-67.
40 Jansen CW, Watson MG. Measurement of range of motion of the finger after flexor tendon repair in zone II of the hand. J Hand Surg (Am). 1993;18:411-417.
41 So YC, Chow SP, Pun WK, et al. Evaluation of results in flexor tendon surgery: A critical analysis of five methods in ninety-five digits. J Hand Surg (Am). 1990;15:258-264.
42 Tsuge K, Ikuta Y, Matsuishi Y. Intra-tendinous tendon suture in the hand—a new technique. Hand. 1975;7:250-255.
43 Tsuge K, Ikuta Y, Matsuishi Y. Repair of flexor tendons by intratendinous suture. J Hand Surg. 1977;2:436-440.
44 Silfverskiöld KL, May EJ. Flexor tendon repair in zone II with a new suture technique and an early mobilization program combining passive and active flexion. J Hand Surg (Am). 1994;19:53-60.
45 Savage R. The influence of wrist position on the minimum force required for active movement of the interphalangeal joints. J Hand Surg (Br). 1988;13:262-268.
46 Dowd MB, Figus A, Harris SB, et al. The results of immediate re-repair of zone 1 and 2 primary flexor tendon repairs which rupture. J Hand Surg (Br). 2006;31:507-513.
47 Peck FH, Kennedy SM, Watson JS, et al. An evaluation of the influence of practitioner-led hand clinics on rupture rates following primary tendon repair in the hand. Br J Plast Surg. 2004;57:45-49.
48 Elliot D. Primary flexor tendon repair: operative repair, pulley management and rehabilitation. J Hand Surg (Br). 2002;27:507-513.
49 Tang JB. Indications, methods, postoperative motion and outcome evaluation of primary flexor tendon repairs in Zone 2. J Hand Surg (Eur). 2007;32:118-129.
50 Moiemen NS, Elliot D. Primary flexor tendon repairs in zone 1. J Hand Surg (Br). 2000;25:78-84.
51 Murphy FG. Repair of laceration of flexor pollicis longus tendon. J Bone Joint Surg (Am). 1937;19:1121-1123.
52 Sirotakova M, Elliot D. Early active mobilization of primary repairs of the flexor pollicis longus tendon. J Hand Surg (Br). 1999;24:647-653.
53 Giesen T, Sirotakova M, Elliot D. Flexor pollicis longus primary repair: further experience with the Tang technique and controlled active mobilisation. J Hand Surg (Eur). 2009;34:758-761.
54 Sirotakova M, Elliot D. Early active mobilization of primary repairs of the flexor pollicis longus tendon with two Kessler two strand core sutures and a strengthened circumferential suture. J Hand Surg (Br). 2004;29:531-535.
55 Smith AM, Evans DM. Biomechanical assessment of a new type of flexor tendon repair. J Hand Surg (Br). 2001;26:217-219.
56 Silfverskiöld KL, Andersson CH. Two new methods of tendon repair: an in vitro evaluation of tensile strength and gap formation. J Hand Surg (Am). 1993;18:58-65.
57 Tang JB, Shi D, Gu YQ, et al. Double and multiple looped suture tendon repair. J Hand Surg (Br). 1994;17:699-703.
58 Tang JB, Gu YT, Rice K, et al. Evaluation of four methods of flexor tendon repair for postoperative active mobilisation. Plast Reconstr Surg. 2001;107:742-749.
59 Kubota H, Aoki M, Pruitt DL, et al. Mechanical properties of various circumferential tendon suture techniques. J Hand Surg (Br). 1996;21:474-480.
60 Wong JK, Cerovac S, Ferguson MW, et al. The cellular effect of a single interrupted suture on tendon. J Hand Surg (Br). 2006;31:358-367.
C The Mayo Clinic Experience
Outline
Many basic science studies have looked at various biomechanical aspects of tendon repair, as well as biologic aspects of tendon healing and adhesion formation.1–4 Manipulation of these biologic processes, in an effort to speed healing, limit scarring, or both, has come the fore of tendon repair research, as biomechanical gains are providing relatively modest clinical improvements in comparison with initial breakthroughs.5
In our research in flexor tendon repair, we similarly investigated methods of improving healing as well as improving motion and lowering work of flexion. Primarily, in our lab, we have focused on lowering gliding resistance by improving repair methods and by the addition of bioactive compounds to limit scar formation and decrease friction. The results are encouraging and will direct future efforts by serving as benchmark and model for comparison of new techniques.6–9
Operative Techniques
There are currently 12 hand surgeons at the Mayo Clinic. Although there are some shared practices, each surgeon manages his or her own patients. As time has progressed, there has been a trend toward repair techniques with greater numbers of core suture strands.2 Currently, for flexor digitorum profundus (FDP) repairs in zone 2, many surgeons use a double Tsuge repair of 3-0 Supramid, with a running peripheral suture of 6-0 Prolene (Figure 13[C]-1). Others prefer a double modified Pennington repair of 3-0 or 4-0 Ticron, Supramid, or, more recently, Fiberwire, again with a running suture of 6-0 Prolene in the epitenon (Figure 13[C]-2). Less commonly, some surgeons use the Strickland six-strand technique, a Tajima modification of a Kessler two-strand technique, or a cruciate four-strand technique.13 We find the locking loops of the Pennington repair to be particularly effective, especially when they are tightened separately prior to tying the knot. For those who use it, the speed of the Tsuge repair is an attraction, especially in cases of polytrauma.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree