Chemokines: Introduction
|
Introduction
The skin is an organ in which the migration, influx, and egress of leukocytes occur in both homeostatic and inflammatory processes. Chemokines and their receptors are accepted as vital mediators of cellular trafficking. Since the discovery of the first chemoattractant cytokine or chemokine in 1977, 50 additional new chemokines and 17 chemokine receptors have been discovered. Most chemokines are small proteins with molecular weights in the 8–10 kDa range and are synthesized constitutively in some cells and can be induced in many cell types by cytokines. Initially associated only with recruitment of leukocyte subsets to inflammatory sites,1 it has become clear that chemokines play roles in angiogenesis, neural development, cancer metastasis, hematopoiesis, and infectious diseases. This chapter will focus primarily on the function of chemokines in inflammatory conditions, but will also touch upon the role of these molecules in other settings as well.
An overview of the structure of chemokines and chemokine receptors will be provided that will detail the molecular signaling pathways initiated by the binding of a chemokine to its cognate receptor. Expression patterns of chemokine receptors will be detailed because of the many types of immune cells that potentially can be recruited to skin under inflammatory conditions. Individual chemokine receptors will be highlighted in regard to biologic function, including facilitation of migration of effector T cells into the skin and the egress of antigen-presenting cells out of the skin. Finally, the roles of chemokines and their receptors in several cutaneous diseases—atopic dermatitis, psoriasis, cancer, and infectious disease—provide a better idea of the diversity of chemokine function in skin.
Structure of Chemokines
Chemokines are grouped into four subfamilies based on the spacing of amino acids between the first two cysteines. The CXC chemokines (also called α-chemokines) show a C–X–C motif with one nonconserved amino acid between the two cysteines. The other major subfamily of chemokines lacks the additional amino acid and is termed the CC subfamily (or β-chemokines). The two remaining subfamilies contain only one member each: the C subfamily is represented by lymphotactin, and fractalkine is the only member of the CXXXC (or CX3C) subfamily. Chemokines can also be assigned to one of two broad and, perhaps, overlapping functional groups. One group (e.g., RANTES, MIP-1α/β LARC, etc.) mediates the attraction and recruitment of immune cells to sites of active inflammation while other (e.g., SLC and SDF-1) appear to play a role in constitutive or homeostatic migration pathways.2
The complexity and redundancy in the nomenclature of chemokines has led to the proposal for a systematic nomenclature for chemokines based on the type of chemokine (C, CXC, CX3C, or CC) and a number based on the order of discovery as proposed by Zlotnik and Yoshie.2 For example, stromal-derived factor-1 (SDF-1), a CXC chemokine, has the systematic name CXCL12. Because both nomenclatures are still in wide use, the original names (abbreviated in most cases) as well as systematic names will be used interchangeably throughout the chapter. Table 12-1 provides a list of chemokine receptors of interest in skin that are discussed in this chapter as well as the major chemokine ligands that bind to them.
Chemokine Receptor | Chemokine Ligand | Expression Pattern | Comments | References |
|---|---|---|---|---|
CCR1 | MIP-1α (CCL3), RANTES (CCL5), MCP-3 (CCL7) | T, Mo, DC, NK, B | Migration of DC and monocytes; strongly upregulated in T cells by IL-2 | |
CCR2 | MCP-1 (CCL2),-3,-4 (CCL13) | T, Mo | Migration of T cells to inflamed sites; replenish LC precursors in epidermis; involved in skin fibrosis via MCP-1 | |
CCR3 | Eotaxin (CCL11) >RANTES, MCP-2 (CCL8),3,4 | Eo, Ba, Th2, K | Migration of Th2 T cells and “allergic” immune cells | |
CCR4 | TARC (CCL17), MDC (CCL22) | T (benign and malignant) | Expression in Th2 > Th1 cells; highly expressed on CLA+ memory T cells; TARC expression by keratinocytes may be important in atopic dermatitis; may guide trafficking of malignant as well as benign inflammatory T cells | |
CCR5 | RANTES, MIP-1α,β (CCL3,4) | T, Mo, DC | Marker for Th1 cells; migration to acutely inflamed sites; may be involved in transmigration of T cells through endothelium; major HIV-1 fusion coreceptor | |
CCR6 | LARC (CCL20) | T, DC, B | Expressed by memory, not naive, T cells; possibly involved in arrest of memory T cells to activated endothelium and recruitment of T cells to epidermis in psoriasis | |
CCR7 | SLC (CCL21), ELC (CCL19) | T, DC, B, melanoma cells | Critical for migration of naive T cells and “central memory” T cells to secondary lymphoid organs; required for mature DC to enter lymphatics and localize to lymph nodes; facilitates nodal metastasis | |
CCR9 | Thymus-expressed chemokine (CCL25) | T, melanoma cells | Associated with melanoma small bowel metastases | |
CCR10 | CTACK (CCL27) | T (benign and malignant), melanoma cells | Preferential response of CLA+ T cells to CTACK in vitro; may be involved in T cell (benign as well as malignant) homing to epidermis, where CTACK is expressed; survival of melanoma is skin | |
CXCR1,2 | IL-8 (CXCL8), MGSA/GRO α (CXCL1), ENA-78 (CXCL5) | N, NK, En, melanoma cells | Recruitment of neutrophils (e.g., epidermis in psoriasis); may be involved in angiogenesis; melanoma growth factor | |
CXCR3 | IP-10 (CXCL10), Mig (CXCL9), I-TAC (CXCL11) | T | Marker for Th1 Cells and may be involved in T cell recruitment to epidermis in CTCL; induces arrest of activated T cells on stimulated endothelium | |
CXCR4 | SDF-1α,β (CXCL12) | T, DC, En, melanoma cells | Major HIV-1 fusion coreceptor; involved in vascular formation; involved in melanoma metastasis to lungs | |
CX3CR1 | Fractalkine (CX3CL1) | T, Mo, MC, NK | May be involved in adhesion on activated T cells, Mo, NK cells to activated endothelium |
Chemokines are highly conserved and have similar secondary and tertiary structure. Based on crystallography studies, a disordered amino terminus followed by three conserved antiparallel β-pleated sheets is a common structural feature of chemokines. Fractalkine is unique in that the chemokine domain sits atop a mucin-like stalk tethered to the plasma membrane via a transmembrane domain and short cytoplasmic tail.30 Although CXC and CC chemokines form multimeric structures under conditions required for structural studies, these associations may be relevant only when chemokines associate with cell-surface components such as glycosaminoglycans (GAGs) or proteoglycans. Since most chemokines have a net positive charge, these proteins tend to bind to negatively charged carbohydrates present on GAGs. Indeed the ability of positively charged chemokines to bind to GAGs is thought to enable chemokines to preferentially associate with the lumenal surface of blood vessels despite the presence of shear forces from the blood that would otherwise wash the chemokines away.
Chemokine Receptors and Signal Transduction
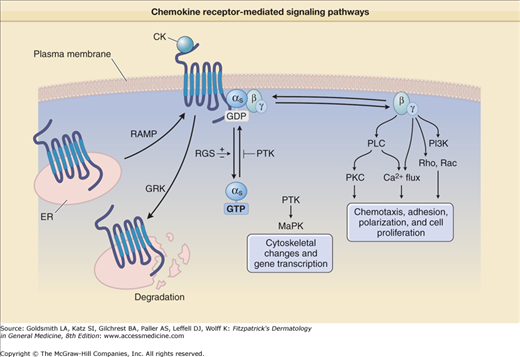
Chemokine receptors are seven transmembrane spanning membrane proteins that couple to intracellular heterotrimeric G-proteins containing α, β, and γ subunits.2 They represent a part of a large family of G-protein coupled receptors (GPCR), including rhodopsin, that have critical biologic functions. Leukocytes express several Gα protein subtypes: s, i, and q, while the β and γ subunits each have 5 and 11 known subtypes, respectively. This complexity in the formation of the heterotrimeric G-protein may account for specificity in the action of certain chemokine receptors. Normally G-proteins are inactive when GDP is bound, but they are activated when the GDP is exchanged for GTP (Fig. 12-1). After binding to a ligand, chemokine receptors rapidly associate with G-proteins, which in turn increases the exchange of GTP for GDP. Pertussis toxin is a commonly used inhibitor of GPCR that irreversibly ADP-ribosylates Gα subunits of the αi class and subsequently prevents most chemokine receptor-mediated signaling.
Figure 12-1
Chemokine receptor-mediated signaling pathways. RAMP = receptor-activity-modifying protein; RGS = regulator of G-protein signaling; GRK = G-protein coupled receptor kinase; DG = 1,2-diacylglycerol; PLC = phospholipase C; PIP2 = phosphatidylinositol-4,5-bisphosphate; IP3 = inositol-1,4,5-triphosphate; PKC = protein kinase C; CK = chemokine; PTX = pertussis toxin; ER = endoplasmic reticulum; PTK = protein tyrosine kinase(s); MAPK = Mitogen activated protein kinase.
Activation of G-proteins leads to the dissociation of the Gα and Gβγ subunits (Fig. 12-1). The Gα subunit has been observed to activate protein tyrosine kinases and mitogen-activated protein kinase, leading to cytoskeletal changes and gene transcription. The Gα subunit retains GTP, which is slowly hydrolyzed by the GTPase activity of this subunit. This GTPase activity is both positively and negatively regulated by GTPase-activating proteins [also known as regulator of G-protein signaling (RGS) proteins]. The Gβγ dimer initiates critical signaling events in regard to chemotaxis and cell adhesion. It activates phospholipase C (PLC)32 leading to formation of diacylglycerol (DAG) and inositol triphosphate [Ins(1,4,5)P3]. Ins(1,4,5)P3 stimulates Ca2+ entry into the cytosol, which along with DAG, activates protein kinase C isoforms. While the Gβγ subunits have been shown to be critical for chemotaxis, the Gαι subunit has no known role in chemotactic migration. There is also evidence that binding of chemokine receptors results in the activation of other intracellular effectors including Ras and Rho, phosphatidylinositol-3-kinase [PI(3)K].33
RhoA and protein kinase C appear to play a role in integrin affinity changes, while PI(3)K may be critical for changes in the avidity state of LFA-1. Other proteins have been found that regulate the synthesis, expression, or degradation of G-protein coupled receptors. For example, receptor-activity-modifying proteins (RAMPS) act as chaperones of seven transmembrane spanning receptors and regulate surface expression as well as the ligand specificity of chemokine receptors (Fig. 12-1). Importantly, after chemokine receptors are exposed to appropriate ligands, they are frequently internalized, leading to an inability of the chemokine receptor to mediate further signaling. This downregulation of chemokine function, which has been termed “desensitization,” occurs because of phosphorylation of Ser/Thr residues in the C-terminal tail by proteins termed GPCR kinases (GRK) and subsequent internalization of the receptor (Fig. 12-1). Desensitization may be an important mechanism for regulating the function of chemokine receptors by inhibiting cell migration as leukocytes arrive at the primary site of inflammation.
Chemokines and Cutaneous Leukocyte Trafficking
Generally speaking, chemokines are thought to play at least three different roles in the recruitment of host defense cells, predominantly leukocytes, to sites of inflammation.34 First, they provide the signal or signals required to cause leukocytes to come to a complete stop (i.e., arrest) in blood vessels at inflamed sites such as skin. Second, chemokines have been shown to have a role in the transmigration of leukocytes from the lumenal side of the blood vessel to the ablumenal side. Third, chemokines attract leukocytes to sites of inflammation in the dermis or epidermis following transmigration. Keratinocytes and endothelial cells are a rich source of chemokines when stimulated by appropriate cytokines. In addition, chemokines and their receptors are known to play critical roles in the emigration of resident skin dendritic cells (i.e., Langerhans cells and dermal dendritic cells) from the skin to draining lymph nodes (LN) via afferent lymphatic vessels, a process that is essential for the development of acquired immune responses.
This section will be divided into three subsections. The first will introduce basic concepts of how all leukocytes arrest in inflamed blood vessels prior to transmigration by introducing the multistep model of leukocyte recruitment. The second will detail mechanisms of T cell migration, while the final subsection will focus on the mechanisms by which chemokines mediate the physiological migration of DC from the skin to regional LN.
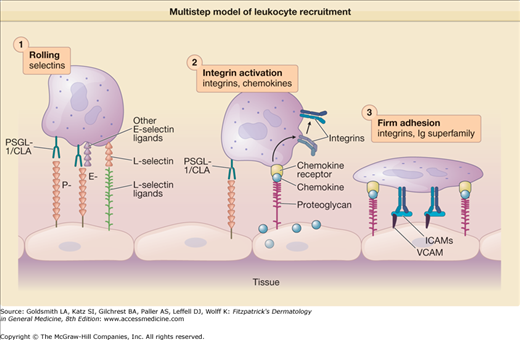
In order for leukocytes to adhere and migrate to peripheral tissues, they must overcome the pushing force of the vascular blood stream as they bind to activated endothelial cells at local sites of inflammation. According to the multistep or cascade model of leukocyte recruitment (Fig. 12-2), one set of homologous adhesion molecules termed selectins mediates the transient attachment of leukocytes to endothelial cells while another set of adhesion molecules termed integrins and their receptors (immunoglobulin superfamily members) mediates stronger binding (i.e., arrest) and transmigration.35 The selectins (E-, L-, and P-selectins) are members of a larger family of carbohydrate-binding proteins termed lectins. The selectins bind their respective carbohydrate ligands located on protein scaffolds and thus mediate the transient binding or “rolling” of leukocytes on endothelial cells.
Figure 12-2
Multistep model of leukocyte recruitment. Leukocytes, pushed by the blood stream, first transiently bind or “roll” on the surface of activated endothelial cells via rapid interactions with P-, E-, or L-selectin. Chemokines are secreted by endothelial cells and bind to proteoglycans that present the chemokine molecules to chemokine receptors on the surface of the leukocyte. After chemokine receptor ligation, intracellular signaling events lead to a change in the conformation of integrins and changes in their distribution on the plasma membrane resulting in “Integrin Activation.” These changes result in high affinity/avidity binding of integrins to endothelial cell intercellular adhesion molecules (ICAMs) and vascular cell adhesion molecule-(VCAM)-1 in a step termed “Firm Adhesion,” which is then followed by transmigration of the leukocyte between endothelial cells and into tissue.
The skin-associated vascular selectin known as E-selectin is upregulated on endothelial cells by inflammatory cytokines such as tumor necrosis factor (TNF)-α and binds to sialyl Lewis x-based carbohydrates. E-selectin ligands form distinct epitopes known as the cutaneous lymphocyte-associated antigen (CLA). CLA is expressed by 10%–40% of memory T cells and has been suggested as a marker for skin-homing T cells.36 At least two chemokine receptors (CCR10 and CCR4) show preferential expression in CLA+ memory T cells.8,20 While E-selectin is likely to be an important component of skin-selective homing, there is also evidence to suggest that L-selectin is involved in T cell migration to skin.37,38
In the second phase of this model, leukocyte integrins such as those of the β2 family must be “turned on” or activated from their resting state in order to bind to their counter receptors such as intercellular adhesion molecule-1 (ICAM-1) that are expressed by endothelial cells. A vast array of data suggest that the binding of chemokines to leukocyte chemokine receptors plays a critical role in activating both β1 and β2 integrins.33,39 Activation of chemokine receptors leads to a complex signaling cascade (Fig. 12-1) that causes a conformational change in individual integrins that leads to increases in the affinity and avidity of individual leukocyte integrins for their ligands. Furthermore, later steps of migration (i.e., transmigration or diapedesis) have been shown to be dependent on chemokines as well in selective cases.13 In the case of neutrophils, their ability to roll on inflamed blood vessels likely depends on their expression of L-selectin and E-selectin ligands while their arrest on activated endothelia likely depends on their expression of CXCR1 and CXCR2 as described below for wound healing. Integrin activation via chemokine-mediated signals appears to be more complex in T cells, which appear to use multiple chemokine receptors, and is described in more detail below.
Antigen-inexperienced T cells are termed naive and can be identified by expressing three cell surface proteins: CD45RA (an isoform of the pan-leukocyte marker), L-selectin, and the chemokine receptor CCR7. These T cells migrate efficiently to secondary LN, where they may make contact with antigen-bearing dendritic cells from the periphery. Once activated by dendritic cells presenting antigen, T cells then express CD45RO, are termed “memory” T cells, and appear to express a variety of adhesion molecules and chemokine receptors, which facilitate their extravasation from blood vessels to inflamed peripheral tissue. A specific subset of CCR7−, L-selectin memory T cells has been proposed to represent an effector memory T cell subset that is ready for rapid deployment at peripheral sites in terms of their cytotoxic activity and ability to mobilize cytokines.14
Although chemokines are both secreted and soluble, the net positive charge on most chemokines allows them to bind to negatively charged proteoglycans such as heparin sulfate that are present on the lumenal surface of endothelial cells, thus allowing them to be presented to T cells as they roll along the lumenal surface (Fig. 12-2). After ligand binding, chemokine receptors send intracellular signals that lead to increases in the affinity and avidity of T-cell integrins such as LFA-1 and VLA-4 for their endothelial receptors ICAM-1 and VCAM-1, respectively.40 Only a few chemokine receptors (CXCR4, CCR7, CCR4, and CCR6) are expressed at sufficient levels on resting peripheral blood T cells to mediate this transition. With activation and IL-2 stimulation, increased numbers of chemokine receptors (e.g., CXCR3) are expressed on activated T cells, making them more likely to respond to other chemokines. In several different systems, inhibition of specific chemokines produced by endothelial cells or chemokine receptors found on T cells dramatically influences T cell arrest in vivo and in vitro.41
CXCR3 serves as a receptor for chemokine ligands Mig, IP-10, and I-Tac. All three of these chemokines are distinguished from other chemokines by being highly upregulated by interferon-γ. Resting T cells do not express functional levels of CXCR3, but upregulate this receptor with activation and cytokines such as IL-2. Once expressed on T cells, CXCR3 is capable of mediating arrest of memory T cells on activated endothelial cells.27 The expression of its chemokine ligands is strongly influenced by the cytokine interferon-γ, which synergistically works with proinflammatory cytokines such as TNF-α to increase expression of these ligands by activated endothelial cells27 and epithelial cells.
In general, activation of T cells by cytokines such as IL-2 is associated with the enhanced expression of CCR1, CCR2, CCR5, and CXCR3. Just as Th1 and Th2 (T cell) subsets have different functional roles, it might have been predicted that these two subsets of T cells would express different chemokine receptors. Indeed, CCR49,42,43 and CCR36 are associated with Th2 cells in vitro while Th1 cells are associated with CCR5 and CXCR3.44
In some instances, chemokine receptors may be regarded as functional markers that characterize distinct T helper cell subsets while also promoting their recruitment to inflammatory sites characterized by “allergic” or “cell-mediated” immune responses, respectively. When T cells are activated in vitro in the presence of Th1-promoting cytokines, CXCR3 and CCR5 appear to be highly expressed, while in the presence of Th2-promoting cytokines, CCR4, CCR8, and CCR3 expression predominates. In rheumatoid arthritis, a Th1-predominant disease, many infiltrating T cells express CCR5 and CXCR345 whereas, in atopic disease, CCR4 expressing T cells may be more frequent.9 CCR6 has recently been described as a marker for a newly characterized T-helper subset, expressing the hallmark effector cytokines IL-17 and IL-22.46 These so-called Th17 cells play a central role in the pathogenesis of psoriasis and other chronic inflammatory autoimmune diseases.47 However, in normal skin, the majority of skin resident T cells also coexpress CCR6, suggesting that CCR6 and CCL20 interactions regulate T cell infiltration in the skin under inflammatory as well as homeostatic conditions.48
While certain chemokine receptors characterize distinct T-cell subsets, flexible regulation of their expression may increase the migratory potential of circulating T cells to diverse tissues. For example, under some conditions, both Th1 and Th2 type T cells can express CCR4.43 Similarly, T regulatory cells (Treg) and Th17 cells share chemokines receptors with other T cell lineages but may alter their chemokine receptor expression profiles, depending on the microenvironment in which they are activated.49
The epidermis is a particularly rich source of chemokines, including RANTES, MIP-3α (CCL20), MCP-1, IP-10, IL-8, LARC, and TARC, which likely contribute to epidermal T cell migration. Keratinocytes from patients with distinctive skin diseases appear to express unique chemokine expression profiles. For instance, keratinocytes derived from patients with atopic dermatitis synthesized mRNA for RANTES at considerably earlier time points in response to IL-4 and TNF-α in comparison to healthy individuals and psoriatic patients.50 Keratinocytes derived from psoriatic patients synthesized higher levels of IP-10 with cytokine stimulation as well as higher constitutive levels of IL-8,50 a chemokine known to recruit neutrophils. IL-8 may contribute to the large numbers of neutrophils that localize to the suprabasal and cornified layers of the epidermis in psoriasis. IP-10 may serve to recruit activated T cells of the Th1 helper phenotype to the epidermis and has been postulated to have a role in the recruitment of malignant T cells to the skin in cutaneous T cell lymphomas.28
CTACK/CCL27 is selectively and constitutively expressed in the epidermis, and its expression is only marginally increased under inflammatory conditions.21 Interestingly, CTACK has been reported to preferentially attract CLA+ memory T cells in vitro21 and has been demonstrated to play a role in the recruitment and function of skin-homing T cells in inflammatory disease models.51,52
Antigen-presenting cells, including dendritic cells (DC) of the skin, are critical initiators of immune responses and their trafficking patterns are thought to influence immunological outcomes. Their mission includes taking up antigen at sites of infection or injury and bringing these antigens to regional LN where they both present antigen and regulate the responses of T and B cells. Skin-resident DCs are initially derived from hematopoietic bone marrow progenitors53 and migrate to skin during the late prenatal and newborn periods of life. Under resting (steady state) conditions, homeostatic production by keratinocytes of CXCL14 (receptor unknown) may be involved in attracting CD14+ DC precursors to the basal layer of the epidermis.54 Similarly, Langerhans cells (LC) as well as CD1c+ LC precursors are strongly chemoattracted to keratinocyte-derived CCL20.55 Under inflammatory conditions, when skin-resident DC and LC leave the skin in large numbers, keratinocytes release a variety of chemokines, including CCL2 and CCL7 (via CCR2)4 and CCL20 (via CCR6),56 which may attract monocyte-like DC precursors to the epidermis in order to replenish the LC population.
When activated by inflammatory cytokines (e.g., TNF-α and IL-1β), lipopolysaccharide, or injury, skin DC, including LC, leave the epidermis, enter afferent lymphatic vessels, and migrate to draining regional LN where they encounter both naive and memory T cells. Chemokines guide the DC on this journey. Activated DC specifically upregulate expression of CCR7, which binds to secondary lymphoid tissue chemokine (SLC/CCL21), a chemokine expressed constitutively by lymphatic endothelial cells15,57 (eFig. 12-2.1). SLC guides DC into dermal lymphatic vessels and helps retain them in SLC-rich regional draining LN (Fig. 12-3
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree