(1)
Department of Clinical Immunology, L. Hirszfeld Institute of Immunology and Experimental Therapy Polish Academy of Sciences, Wroclaw, Poland
Abstract
Vascularized composite allograft (VCA) contains multiple tissue components with tissue-resident cells and unique immunologic characteristics that ultimately contribute to the chimerism induction and successful outcome of the VCA. Complexity of transplanted tissue components in different VCA models determines the quality and robustness of donor-specific chimerism. As introduced in experimental studies, vascularized bone containing hematopoietic cells constitute an integral part of some VCA including limb and face transplant models. Transplantation of vascularized bone with their natural microenvironment containing bone marrow of donor-origin as an integral part of VCA, facilitate donor cell engraftment and chimerism maintenance.
In this Chapter, based on our own experience, we have analyzed the impact of vascularized bone marrow transplantation for chimerism induction as prerequisite for tolerance induction in VCA in the context of immunomodulatory protocols and VCA complexity.
Keywords
Vascularized Composite Allograft (VCA)Vascularized bone marrow transplantationChimerismTransplantation toleranceLimb allograftFace transplantAbbreviations
ALS
Anti-lymphocyte serum
BMC
Bone marrow cells
BMT
Bone marrow transplantation
CsA
Cyclosporine A
GvHD
Graft-versus-host disease
IBOMC
Iliac bone osteomusculocutaneous
LBN
Lewis-Brown Norway
mAb
Monoclonal antibody
MHC
Major histocompatibility complex
MLR
Mixed lymphocyte reaction
TCR
T-cell receptor
VBMT
Vascularized bone marrow transplants
VCA
Vascularized composite allograft
Introduction
Hematopoietic cells of donor-origin have a great potential for tolerance induction in solid organs and in vascularized composite allografts (VCA). In solid organ transplants donor bone marrow cells (BMC) constitute a part of supportive therapy along with or after organ transplantation, whereas in VCA donor BMC usually constitute an integral part of transplanted tissues containing vascularized bone with BMC and thus are considered potentially tolerogenic.
Experimental limb allograft and face transplant models carrying bone component containing BMC are an examples of vascularized bone marrow transplants (BMT). These models function as vascularized carrier of donor BMC, providing a continuous source of donor hematopoietic cell delivery and are contributing to chimerism development and maintenance [1–5]. Vascularized bone marrow transplants serve as an experimental model to study feasibility and applicability of augmentative potential of vascularized BMT for chimerism induction in VCA models. Various models of vascularized BMT have been developed in the Cleveland Clinic Laboratory of Microsurgery including limb, sternum, femoral bone marrow transplant, iliac bone and bone components of face transplant models including calvaria, maxilla, and mandible [2–9]. Transplantation of vascularized bone marrow meets the criteria for chimerism induction without the need for host conditioning. This is possible since in vascularized BMT, hematopoietic stem cells are transplanted within their natural microenvironment. This technique does not require bone marrow processing and permits donor cell engraftment.
Experimental models of vascularized BMT were created not only to assess the surgical and functional recovery after transplantation, but also to assess tolerance-inducing strategies based on immunomodulatory protocols which will have potential application in the clinic. The efficacy of chimerism induction via vascularized BMT was tested based on different immunosuppressive protocols used in the Microsurgery Laboratory: (i) monotherapy protocol with calcineurin inhibitors and (ii) immunodepletive protocol of antilymphocye serum (ALS) or monoclonal antibody (mAb) anti αβ-T-cell receptor (anti αβ-TCRmAb) combined with cyclosporine A (CsA) therapy.
The Multi-Tissue Models Containing Vascularized Bone Marrow
Complexity of the VCA introduces surgical and immunological challenges and requires adjustment of immunosuppressive protocols. In most clinical applications, such as hand and face transplants, VCA contains multi-tissue components including skin, subcutaneous tissue, muscle, bone with bone marrow, lymph nodes, nerve, tendon and mucosa [10].
The Limb Allograft as Vascularized BMT Model
The most commonly used experimental model of vascularized BMT is functional orthotopic limb allograft model. The limb allograft model was created not only on improvement of microsurgical techniques but also to test tolerance-inducing protocols. The limb represents a specific model of the vascularized BMT since vascularized bone, with BMC, in addition to muscles, skin, nerves and tendons, constitutes a structural component of the VCA [4, 11, 12]. We have shown that a limb allograft contains approximately 50 × 106 of the bone marrow cells which may play a significant role in chimerism induction [8].
CsA Monotherapy Protocol in Limb Allograft Model
Our first studies on limb allografts were performed between Lewis-Brown-Norway (LBN) (RT1l+n) donors and Lewis (RT1l) recipients under combined protocol of systemic CsA (4 mg/kg/day) monotherapy with topical application of fluocinolone acetonide (6 mg/cm2/day) both started at the day of surgery and maintained during entire follow-up period [13]. Synergistic therapeutic effect of low dose of CsA and topical application of steroids allowed for extended limb allograft survival, up to 51 days. Our experience with limb allograft model under continued CsA monotherapy resulted in prolonged allograft survival, however, after CsA treatment cessation limb allografts were rejected.
To make immunosuppressive protocol clinically applicable experimental models of vascularized BMT for mixed chimerism and transplantation tolerance induction was developed under transient nonselective or selective immunodepletion and short-term immunsuppression, without lethal conditioning.
Immunodepletive Protocols in Limb Allograft Model
Elimination of memory T lymphocytes or inhibition of T cell activation constitutes a critical mechanism of transplantation tolerance. The non-selective depletion of T cells is the most widely used protocol in many experimental models and in the clinic, and is accomplished by targeting of all T cells, not only alloreactive T cells, by either polyclonal (antilymphocyte sera) or monoclonal antibodies (mAb) (anti-CD3, anti-CD52) [14].
Working on tolerance-inducing strategies we created limb allograft model, and tested a 21-day combined protocol of ALS and CsA therapy for chimerism induction. Transplantations were performed in semiallogenic rat model between (LBN) (RT1l+n) donors and Lewis (RT1l) recipients. The combined immunodepletive protocol of ALS and CsA significantly prolonged limb allograft survival (over 420 days) compared to monotherapy with ALS or CsA alone (6 and 23 days respectively) and this was associated with the presence of donor-specific hematopoietic chimerism in the peripheral blood ranging from 35 to 42 %, whereas in non-tolerant animals chimerism was not detected [4].
After achieving success in tolerance induction in semi-allogenic limb transplant model, we applied the immunodepletive protocol of ALS and CsA to a more immunogenetically challenging model in fully MHC mismatched animals [BN(RT1n) donors and Lewis (RT1l) recipients]. Under ALS/CsA protocol, limb allograft survival was extended by up to 56 days; however, tolerance was not achieved [15]. Only transient, donor-derived chimerism (17 ± 1.1 % at day 35) was detected, and dropped down to 0 at the time of rejection. This study confirmed that transplantation across a strong MHC barrier mandates adjustments in immunosuppressive protocols.
To further expand our investigation of tolerance inducing protocol we have developed new strategy with selective targeting of potentially alloreactive T-cells in limb transplant model. Early studies using mAb against αβ-TCR introduced by Heidecke et al. in a rat heart allograft model documented long-term cardiac allograft survival after pre-transplant treatment with monoclonal antibody R73 (mouse anti-rat αβ-TCR) [16]. Selective depletion of alloreactive T-cells reduces initial alloreactive response by inhibition of specific antigenic peptides such as αβ-TCR which delivers first signal of activation. Moreover, anti-αβ-TCR mAb is selectively targeting alloreactive T-cells only, but not affects other T-cells such as γδ T-cells, natural killer (NK) cells and other leukocytes including monocytes, thus preventing innate immunity [17].
Initial studies on limb allograft model tested the dose and duration of anti αβ-TCR mAb CsA therapy and resulted in establishment of dose of anti αβ-TCR monoclonal antibody, at 50 μg/day, in combination with tapered dose of CsA, from 16 to 2 mg/kg/day, over 35-days post-transplant under this protocol [18]. Limb allograft survival (over 720 days) was associated with the presence of donor-specific chimerism in CD4 (6.7 %) and CD8 (1.2 %) of T cell subpopulations. In contrast, a 35-day protocol of CsA monotherapy resulted in limb allograft rejection within 2 weeks after immunosuppression cessation.
To further test the efficacy of short-term anti αβ-TCR/CsA protocol, we investigated the effect of different treatment intervals (21, 7, and 5 days) protocols for chimerism development, allograft survival and tolerance induction [19]. Indefinite limb allograft survival and functional recovery was associated with the presence of a stable level of donor-specific chimerism ranging from 10 to 12 % in CD4, and 6 to 9 % in CD8 T cell subpopulation. In this study, a combined anti αβ-TCR/CsA protocol resulted in over 95 % depletion of αβ-TCR positive cells at, as early as, post-transplant day 7, and T cell repopulation was present at 35 days after treatment cessation. The timing of deletional effect under 5 day protocol correlates with the maturation process of newly developed T cells (both from the donor and the recipient) in thymus, which takes approximately 28 days, and thus the short period of immunodepletion is sufficient to create a chronological window of unresponsiveness to the new repertoire of T lymphocytes [19]. We have confirmed that 5, 7, and 21-day immunodepletive protocols with anti αβ-TCR/CsA resulted in long-term limb allograft survival, and we have chosen 7-day therapy as a standard immunodepletive protocol for tolerance induction in VCA. The rationale to choose 7-day protocol of αβ-TCR/CsA is the opportunity to use this protocol at the day of transplantation without recipient preconditioning and this has the advantages of direct clinical application since in clinical VCA a preconditioning protocol rather will never be accepted [20].
Central (intrathymic) clonal deletion provides a robust form of tolerance in all chimerism-related approaches, even to the most immunogenic tissue, such as skin. Clonal deletion is usually considered superior to regulatory or anergic mechanisms since clonal deletion physically eliminates T cells with certain specificity [21, 22]. To assess the role of thymus in tolerance induction in VCA, a series of experiments were designed using 7-day combined immunosuppressive protocol of anti-αβ-TCRmAb/CsA in rat limb allograft model [12]. Allotransplants were performed between semi-allogenic LBN donors and euthymic and thymectomized Lewis rat recipients without maintenance therapy. Treatment with αβ-TCR/CsA resulted in indefinite limb allograft survival (median survival time (MST) = 370 days) in euthymic recipients; however, combined protocol of anti αβ-TCR/CsA applied to thymectomized Lewis recipients prolonged MST of limb allograft for only 51 days. In tolerogenic animals stable T-cell chimerism of donor-origin was achieved at 17.3 % for CD4 and 13.9 % for CD8, in euthymic rats, whereas only transient chimerism, 7–9 % for CD4 and 2–4 % for CD8 T cells, was detected in the thymectomized rats. Engraftment of donor-origin cells into lymphoid organs (spleen, lymph nodes and thymus) of the recipients in the euthymic rats under anti αβ-TCR/CsA protocol was confirmed. In contrast, in thymectomized limb allograft recipients, donor-origin cells were detected in the spleen and lymph nodes at the time of anti αβ-TCR/CsA immunosuppressive protocol cessation, but were absent in the lymph nodes, and only scattered cells were found in the spleen, at the time of allograft rejection.
This study confirmed that mixed chimerism ensures intrathymic T cell deletion of donor-reactive cells, as long as chimerism persists. This is mediated mainly by bone marrow derived dendritic cells of both donor and recipient origin. In this limb allograft model, the constant delivery of bone marrow cells of donor origin was permitted from the transplanted limb containing both the femoral and tibial bone with hematopoietic cells. Mixed chimerism provides cells with an antigen-presenting function of both the donor and recipient acting in the periphery, and preserving recipient’s immunocompetence to the third party antigens. Based on these observations, we suggest that nonmyeloablative 7-day protocol of selective targeting of αβ-TCR positive cells, in combination with CsA therapy, may facilitate engraftment of donor cells into the thymus, leading to negative selection of newly developing alloreactive host T cells. Both a central and peripheral mechanism may be involved in chimerism maintenance and tolerance to limb allograft.
A successful protocol of combined anti αβ-TCR/CsA with selective depletion of potentially alloreactive T cells was also applied in a fully MHC mismatched rat limb allograft model performed between BN(RT1n) donors and Lew(RT1l) recipients, making this short-term, nonmyeloablative VCA conditioning, clinically applicable. Tolerance to the limb allograft was associated with stable, multilineage, donor-specific chimerism in T cell population: CD4 (7.6 %) and CD8 (1.3 %), and chimerism maintenance was supported by B cell lineage (16.5 % of RT1n/CD45RA) [11] (Fig. 73.1).
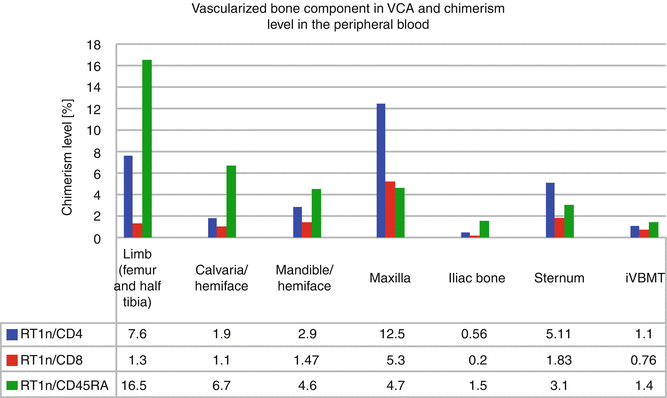

Fig. 73.1
Chimerism level in the peripheral blood of recipients of vascularized composite allograft containing vascularized bone component with hematopoietic cells
In all our experimental limb allograft model, Tolerance was confirmed ex vivo by MLR assay showing hyporesponsiveness to the donor antigens, and in vivo by acceptance of donor skin grafts [4, 11, 12, 18].
In limb allograft models, a vascularized bone component containing BMC of donor origin contributed to long-term allograft survival. Following revascularization, BMC migrated from the transplanted VCA and engrafted and repopulated in different tissues of the limb recipients, including the recipient’s bone marrow compartment and as such, contributed to chimerism maintenance.
Face Allograft Model Containing Vascularized Bone Component
Vascularized bone component comprise a part of transplanted tissues in face allograft the most complex VCA model. The clinical need to cover extensive craniomaxillofacial defects, including bony and soft tissue components, encouraged us to develop rat model of composite hemiface/calvaria, maxilla and hemiface/mandible/tongue transplantation models [5–7, 23]. These surgically challenging models were kept up under low non-toxic dose of CsA (2 mg/kg/day) monotherapy and were immunologically assessed for the presence of chimerism at different time-point starting from day 7 post-transplant up to the end-point at sacrifice day.
Chimerism in Hemiface/Calvaria Model
To restore the of extensive craniomaxillofacial defects including bone and soft tissues, we have developed rat model of composite hemiface/calvaria allograft [6]. Long-term follow-up, up to 220 days, under low maintenance dose of CsA, and subsequent histological and immunologic assessment, proved viability of bone component of composite hemiface/calvaria allograft with viable BM cells, and the presence of peripheral blood chimerism. In contrast to face transplant model without bone component [24], in hemiface/calvaria model peripheral blood chimerism was supported predominantly by B lymphocyte population (Fig. 73.1) and viable BMC were detected within vascularized bone component [6].
Chimerism in Maxilla Model
Another experimental model tested the application of heterotopic rat maxilla allotransplantation for coverage of midfacial or maxillary deformities [7




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








