Fig. 74.1
(a, b) Intravenous bone marrow nsplantation. (c, d) Intraosseous bone marrow transplantation. Before intraosseous bone marrow transplantation, the same volume of recipients’ bone marrow was withdrawn from the femur cavity to create the ‘space’ for donor bone marrow delivery
Intraosseous BMT
Intraosseous BMT was performed through a lateral femoral incision. A burr hole was created in the bone with a 27 gage needle. Before BMC infusion, the same volume of recipients’ bone marrow (50 μL) was withdrawn from the femur. After the cells were injected into the femur, the hole was sealed with bone wax to prevent leaking. The incision was then closed in a two-layer fashion, including muscle and skin closure (Fig. 74.1c, d).
Effects of Intraosseous Vs Intravenous BMT
In this MHC-mismatch rat model, we have introduced the beneficial effect of intraosseous BMC delivery when compared with standard intravenous BMC transplantation. We revealed that hematopoietic recovery and efficacy of donor-cell engraftment into the bone marrow and lymphoid organ compartments resulted in higher chimerism level after intraosseous BMT (7.9 ± 1.3 %) under αβ-TCR/CsA and 70 × 106 BMC transplantation, whereas lower chimerism (4.2 ± 1.4 %) was observed after intravenous BMT [7]. The seeding efficacy of donor cells into lymphoid tissues, including thymus, was greater after intraosseous BMT when compared with standard i.v. transplantation (p = 0.007) [7].
Administration on donor hematopoietic cells directly into the bone have beneficial effect on chimerism induction because only 10–20 % of hematopoietic stem cells infused intravenously have the ability to reach the bone marrow [10]. The cells which do not enter the bone marrow are trapped by other organs such as liver and lungs, which have large capillary beds. In contrast, direct intra-bone BMC delivery reveals a 15-fold greater potential for hematopoietic stem cell engraftment [11]. Clinical applicability of intraosseous BMC delivery will be accepted in restrictive circumstances: when the number of available stem cells is limited e.g. in the transplantation of cord blood cells [11–13], when transplanting a highly selected cell population [14], in VCA transplants as supportive therapy for inducing donor-specific immune unresponsiveness [15], as a more effective method of co-transplanting hematopoietic and mesenchymal stem cells [16, 17].
Supportive Therapy with Bone Marrow Cells for Face Allograft Survival
Supportive therapy with donor BMT was applied for face transplant model across MHC barrier under 7 day α/β TCR/CsA protocol. At the day of face transplant, unmanipulated donor BMCs were transplanted directly into the femoral bone cavity simultaneously with hemifacial flap transplantation. Preliminary results showed that enhancement protocol with donor BMT for facial VCA resulted in different time of face allograft survival (from 36 to 496 days) in semiallogenic hemiface allograft recipients; however the rate of survival was lower compared to face transplant recipients under low dose of maintenance CsA monotherapy [18, 19]. In long-term survived face transplant recipients augmented with donor BMT and 7 day anti αβ-TCR/CsA protocol chimerism was developed in both the T and B cell populations, however, at long-term follow-up T cell chimerism was low (below 2.0 %) but was maintained in B cell lymphocytes (12.6 %). These observations are consistent with face transplant models containing vascularized bone elements (hemiface/calvaria and hemiface/mandible) with hematopoietic cells of donor origin, where chimerism was maintained by B cell lymphocytes in long-term survived facial VCA recipients [20, 21]. Moreover, long-term face allograft acceptance was also associated with the presence of regulatory T cells (CD4+/CD25+); in tolerant recipients the level of CD4+/CD25+ cells was 50 % higher compared to those which face allograft rejected [18].
Cell-Based Therapy into Immunoprivileged Compartments
The testis, renal capsule and central nervous system represent immunological privileged tissues as evidenced by its ability to support grafts with minimal rejection [22–24]. Immune privilege is essential for the tolerance to alloantigens from transplanted cells or tissues that appear after the constitution of self-tolerance, but also providing efficient protection against pathogens. The immunoprivileged sites are more suitable for cellular transplantation and offer complete or partial protection from allorejection without the need for continuous immunosuppressive therapy. The intrathecal, intracapsular space and intermembrane testis division have been suggested to establish protection to allogeneic cells as immunoprivileged regions.
To compare tolerogenic effects of different strategies of BMT into immunprivileged compartments we have developed rat experimental model of vascularized skin allograft (VSA), performed between fully MHC mismatched rats ACI (RT1a) donors and Lewis (RT1l) recipients, under 7 days of immunodepletive protocol of anti-αβ-TCRmAb/CsA therapy supported with donor BMT into three different immunoprivileged compartments: (1) intracapsular BMC transplantation; (2) intragonadal BMC delivery; and (3) intrathecal (to the cerebrospinal fluid) BMC infusion.
Transplantation Procedure of Vascular Skin Allograft and Donor BMT
The surgical procedure of skin allograft transplantation was performed according previously described technique [4].
Bone marrow cells were isolated as described above. Before BMT, cells were stained with PKH-26 dye to evaluate migration and engraftment into lymphoid and non-lymphoid organs of recipients. At the same time as skin allograft, unselected BMC (100 × 106 cells) were transplanted into different immunoprivileged sites: (1) under right kidney capsule, (2) into right testis between tunica albuginea and seminiferous tubules, and (3) were infused intrathecally.
Bone Marrow Transplantation Technique into Immunoprivileged Compartments
Intracapsular BMC Transplantation
After anesthesia has taken effect, using small incision through skin, muscle and peritoneum of the left back side of the animal we exposed the left kidney outside the body using two saline-wetted cotton-tipped applicators. Employing a slight pressure to both sides of the incision we rolled the kidney out of the abdominal cavity. The kidney capsule was moisten by applying saline with a cotton-tipped swab. Then through the lower pole of the kidney not involving the renal pelvis, 0.06 ml of bone marrow cells suspension was injected under the upper kidney capsule using a 0.5 ml syringe with 31G needle (Fig. 74.2a, b). The bleeding after kidney puncture was stopped using dry cotton tipped swab or cauterization with low heat and the kidney was re-moistened with sterile saline. The kidney was gently replaced into the peritoneum prior to closing the abdominal wall. The muscle layer and the skin were sewed with a running 4-0 absorbable suture.
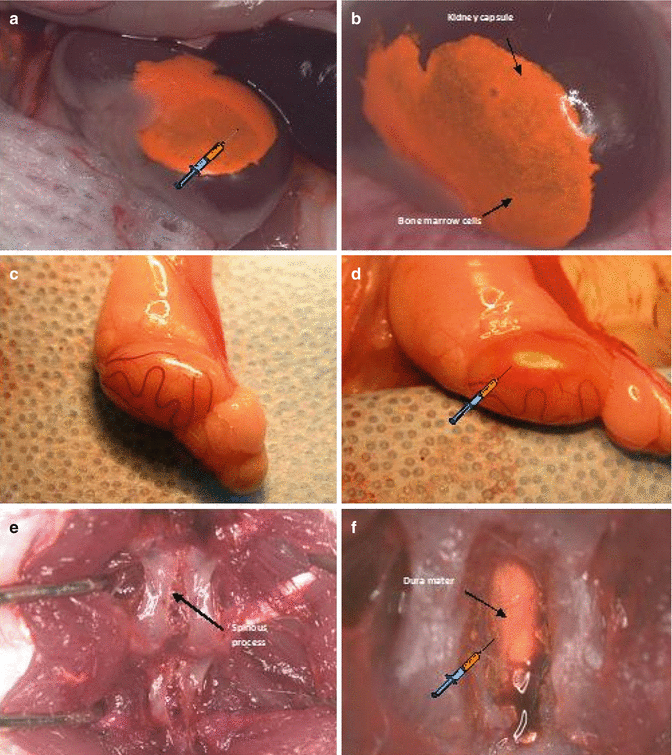

Fig. 74.2
Immunoprivilege sites of bone marrow cells (BMC) 100 × 106 cells transplantation. (a, b) Intracapsular BMC transplantation; (c, d) Intragonadal BMC transplantation; (e, f) Intrathecal BMC transplantation
Intragonadal BMC Transplantation
The animal was placed in lateral supine position. Then the scrotal and inguinal regions were trimmed and swabbed with Povidone-Iodine solution before the surgery. A linear incision was made lateral to the median raphe on a right side. The right testis enclosed in the parietal vaginal tunic was gently exposed using cotton-tipped stick. The small incision helped keeping the testicle raised and exposed. The testicle was swabbed with normal saline preventing tunica albuginea rupture. Using 0.5 ml syringe with 31G needle, 0.06 ml of BMC suspension was injected between tunica albuginea and convoluted seminiferous tubules (Fig. 74.2c, d). After the cells transplantation the testicle was placed back in the scrotum. The scrotum skin was closed with a running stitch using 5-0 running silk sutures on C-6 19 mm needle.
Intrathecal BMC Transplantation
Lie prone position, after hair trimmed and antiseptic decontamination of operation field a posterior midline skin incision was performed approximately 2–3 cm above vertebrae L4-L5. The paraspinous muscles were detached by termocautery technique. Using the Lov-Kerrison punch the laminotomy was conducted. Particular attention was paid to avoid the damage of external vertebral venous plexuses (extraspinal veins) responsible for persistent and massive venous hemorrhage. Afterwards dura mater was exposed and 0.08 ml of bone marrow cells suspension was transplanted using 0.5 ml syringe with 31G needle (Fig. 74.2e, f). Meticulous attention on this stage is required. Rapidly injected fluid into subarachnoid space induces cerebrospinal fluid hypertension which provokes irreversible complications. The needle was gently removed and the needle hole was covered in dura matter with the previously prepared paraspinous muscle patch, silked with bone wax if necessary. Subcutaneous tissue and skin were adapted with running 4-0 absorbable sutures.
Results of BMT into Immunoprivileged Sites
Intracapsular BMC delivery resulted in prolonged skin allograft survival from 28 to 57 days, whereas intragonadal BMT extended skin allograft survival up to 69 days. The most effective for skin allograft survival was intrathecal BMC delivery; animals accepted skin allograft from 50 to 78 days post-transplant [25] (Fig. 74.3). Beneficial effect of intrathecal BMT was associated with higher level of regulatory T cells CD4+/CD25+ in the peripheral blood of the recipients compared to control without BMC and to intragonadal and intracapsular BMC delivery (p < 0.05).
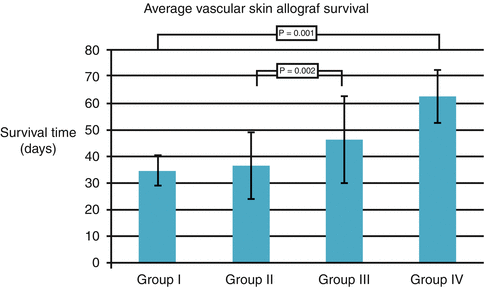

Fig. 74.3
The effect of BMT (100 × 106 cells) into immunoprivilege sites on vascularized skin allograft survival. Group I: Control, without supportive therapy with BMC, Group II: Intracapsular BMC transplantation, Group III: Intragonadal BMC transplantation, Group IV: Intrathecal BMC transplantation
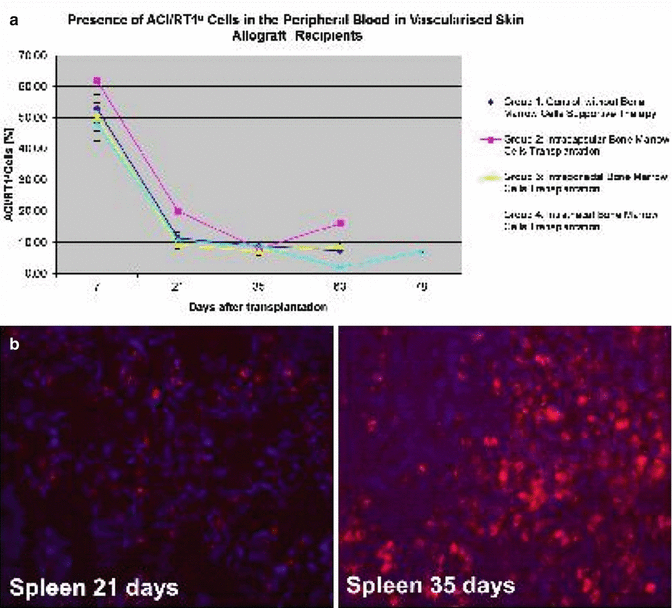
There were no significant differences in peripheral blood chimerism level between experimental groups. The level of donor chimerism for T cells (RT1a/CD4, RT1a/CD8), B-cells (RT1a/CD45RA) and monocyte/granulocyte (RT1a/CD11b/c) was similar in all experimental groups during follow-up period (Fig. 74.4a). Moreover we found that BMC delivery into immunoprivileged sites resulted in donor cell engraftment into peripheral lymphoid organs of recipients [26] (Fig. 74.4b).
Get Clinical Tree app for offline access

Fig. 74.4
(a) Chimerism (ACI/RT1a




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








