Fig. 16.1
Camptodactyly. Non-traumatic progressive flexion deformity typically involving the ulnar digit(s), often with an intrinsic minus posture
The term camptodactyly is Greek for “bent finger.” Ever since Tamplin’s description in 1846 [1], the definition, etiology, and treatment have varied in the literature. Almost every structure around the PIP joint has been implicated, and reconciliation of these pathologic observations has been debated.
Presentation
Camptodactyly affects less than 1 % of the population and is usually asymptomatic [2, 3]. The anomaly generally occurs sporadically; however, it can be inherited in an autosomal dominant pattern with variable expressivity and incomplete penetrance. Bilateral deformities occur in approximately two-thirds of cases and the little finger is almost always involved. Multiple digits can be affected, with less frequent involvement of the radial digits. The thumb is not involved. Metacarpophalangeal (MCP) joint hyperextension can accompany PIP flexion contractures.
When the initial presentation is during infancy, males and females are equally affected. Females are more commonly affected when the initial presentation is during adolescence.
Benson classified patients into three types. Type I is an isolated anomaly of the little finger PIP joint that presents in infancy and can involve up to two fingers. Type II has the same clinical features but presents in adolescence. Type III is characterized by severe contractures, bilateral involvement, multiple digits, and other associated congenital anomalies. Type III camptodactyly presents at birth and is seen as a manifestation of a more generalized condition (Table 16.1).
Table 16.1
Generalized conditions associated with camptodactyly
Conditions | |
|---|---|
Craniofacial | Orofaciodigital syndrome |
Craniocarpotarsal dystrophy (Freeman–Sheldon syndrome) | |
Oculodentodigital dysplasia | |
Chromosomal | Trisomy 13–15 |
Short Stature | Campomelic dysplasia I |
Mucopolysaccharidosis | |
Facial–digital–genital (Aarskog–Scott syndrome) | |
Others | Osteo-onychodysostosis (Turner–Kieser syndrome) |
Cerebrohepatorenal (Zellweger’s syndrome) | |
Jacob-Downey syndrome |
The initial presentation of Type I and Type II camptodactyly at infancy and adolescence, respectively, may be related to growth spurts during which flexor–extensor imbalances manifest.
The PIP flexion deformity may be fixed or passively correctible, which has important treatment implications. Foucher proposed subclassifying Type I and II camptodactyly into “stiff” or “correctable” categories to guide his treatment approach [4].
Pathology
Abnormalities of almost every structure around the PIP joint have been described. Some believe all of these structures are involved but to varying degrees [4, 5]. Others believe that specific abnormalities of the flexor digitorum superficialis (FDS) or the intrinsic musculature [6–8] result in secondary changes with time and growth.
Early descriptions of camptodactyly have noted a tight, contracted, or underdeveloped FDS tendon [1, 9] and suggest that the deformity is the result of an imbalance of flexor and extensor forces. Aberrant FDS tendon origins in the absence of a normal muscle belly have been described and include the A2 pulley, palmar aponeurosis, flexor tendon sheath, and transverse carpal ligament [10].
Surgical explorations by Courtemanche and McFarlane have identified consistent abnormalities of lumbrical muscles, suggesting that these may be the primary etiology of camptodactyly [6–8]. An aberrant lumbrical origin from the flexor retinaculum [11] has also been described. More commonly, aberrant lumbrical insertions have been described and include the FDS tendon [6–8, 12, 13] and MCP joint capsule [6–8]. Inadequate lumbrical muscle function leads to an intrinsic-minus posture, which may lead to the deformity seen with camptodactyly.
Other soft tissue abnormalities include extensor incompetence, collateral ligament contracture, volar plate contracture, and volar skin deficiency [1, 4, 5]. Bony changes can be seen with long-standing deformities [1, 7] and are consistent with growth in the setting of a chronically flexed joint. The head of the proximal phalanx is narrowed in the dorsovolar plane with loss of the normal volar convexity. The articular surface of the middle phalanx base can have a shallow dorsal groove. Radiographic changes were seen in approximately 15 % of patients in McFarlane and Smith’s series [1, 7]. Smith argues that all of these abnormalities are common to all cases of camptodactyly, but to varying degrees. McFarlane argues that aberrations of lumbrical muscle insertion are the unifying cause and all other changes occur secondary to chronic motor imbalance. He points out how previous anatomic studies have demonstrated that normal anatomic variations of the intrinsic muscles occur more frequently in the ulnar digits and that these are the same digits that are involved with camptodactyly.
Diagnosis
Differentiation of camptodactyly from other conditions is accomplished through careful history and physical examination. Camptodactyly is seen in the absence of trauma, inflammation, and palpable lesions. The deformity is slowly progressive and generally occurs in isolation. A trigger finger may be associated with a palpable click on extension. Juvenile palmar fibromatosis or Dupuytren’s disease is associated with palpable subcutaneous nodules. A boutonniere deformity should be associated with antecedent trauma and swelling. Inflammatory arthridities manifest with inflammation and more generalized involvement. Symphalangism is characterized by no active or passive joint motion and an absence of skin creases. Arthrogryposis involves generalized muscular and skeletal deficiencies. Pterygium syndrome is usually associated with involvement of multiple joints.
Following a thorough examination of the upper extremity, active and passive range of motion of the PIP joint should be evaluated and the influence of adjacent joint positions should be noted. FDS tightness can be determined by tenodesis effect in which wrist and MCP extension places the FDS on stretch and results in further loss of passive PIP extension. Intrinsic motor deficiency can be assessed using the Bouvier maneuver, in which active PIP extension is tested with the MCP joint stabilized in flexion. Restoration of full PIP extension suggests that inadequate MCP flexion, via the intrinsic muscles, contributes to the deformity. Extensor competence can be tested using an extensor tenodesis test in which full flexion of the wrist and MCP places the extensor system on stretch. This should result in full PIP extension. Long-standing flexion deformities can result in attenuation of the central slip, in which case PIP extension would not occur with this test. Volar skin deficiency can be determined by testing passive PIP extension with the MCP in flexion and in extension. Blanching and loss of passive PIP extension when the MCP is extended suggests volar soft tissue deficiency.
Flexor digitorum profundus (FDP) and FDS function of each finger should be evaluated. Given that the FDP to each finger acts through a common muscle belly, in order to test isolated FDS function to an individual finger, all other digits must be held in extension while finger flexion of the digit of interest is evaluated. The ring and little finger FDS are conjoined in 30 % of people. If PIP flexion is not possible for the little finger, release of the ring finger should result in PIP flexion of both digits if their FDS is conjoined.
Treatment
Conservative management should be the first line of treatment. PIP contractures of less than 30° rarely have any functional impact, and greater contractures are often well tolerated.
Nonsurgical Management
Methods of nonsurgical treatment include passive stretching, static splinting, dynamic splinting, or any combination of these. Results often rely upon patient and family compliance, but can also vary with patient age and severity of the contracture.
Rhee et al. reported on the results of passive stretching for simple camptodactyly in children younger than 3 years of age [14]. They included 61 digits in 22 patients. Their stretching protocol involved 5 min of passive stretching 20 times per day until the deformity was corrected or the improvement “stabilized,” followed by maintenance stretching 5–10 times per day. Children were stratified according to initial severity of contracture (<30°, 30–60°, >60°) and were found to have significant corrections: 20° to 1° for mild contractures, 39° to 12° for moderate contractures, and 75° to 28° for severe contractures. The mean follow-up was 26 months and the only correlation with degree of improvement was the initial flexion contracture. Although they demonstrated good results in a homogenous group of Korean children with Type I camptodactyly, their protocol is time intensive, requires considerable caregiver effort, and the long-term outcomes, especially regarding the risk of progression or recurrence as children move into adolescence, are unknown.
Benson et al. reported on the results of passive stretching combined with static splinting in patients with all types of camptodactyly. They treated 24 digits in 13 patients with Type I camptodactyly with static splints and daily passive stretching. The splints were initially worn for 15–18 h per day and slowly weaned after full passive motion was achieved. The average correction was −22.9° to −0.9° of passive extension with a mean follow-up of 36 months. They treated five digits in four patients with Type II camptodactyly who had experienced an overall worsening of the flexion contracture. They also treated 30 digits in five patients with Type III camptodactyly with overall improvement from −23° to −1° of passive extension. Most fingers were treated with splinting and the fingers that underwent surgery had significant improvements.
Hori et al. used dynamic splints to treat 34 digits in 24 patients. Treatment involved splinting for 24 h per day during the first few months until full correction was achieved, followed by use of the digit for 8 h per day. Fifteen patients were less than 5 years old and eight patients were older than 10 years of age; however, they did not report results according to camptodactyly type. The average correction of the contractures was 40° to 10° with average follow-up of 56 months (minimum 10 months). Miura et al. also used dynamic splints and found that the results were better in children younger than 5 years than in children who were older than 5 years [15].
Siebert et al. reported results of both nonsurgical and surgical treatment for simple camptodactyly. Nonsurgical treatment involved combinations of passive stretching, static splinting, and dynamic splinting. Although patients were not classified according to age of onset, most children who underwent nonsurgical treatment were close to adolescence. Their results were similar to those reported by Rhee when stratified according to initial contracture severity. The authors found that the overall results of surgical treatment were worse than nonsurgical treatment and suggest that surgery should only be considered with contractures of greater than 60°, after nonsurgical treatment has failed.
Surgical
Surgical treatment of camptodactyly should be reserved for severe cases in which all efforts towards nonsurgical management have failed. The results of surgery are inconsistent and the risk of PIP flexion loss needs to be weighed against the more limited gains in PIP extension. Flexion contractures of up to 60° in the ulnar digits are well tolerated and should not be treated surgically. Postoperative rehabilitation is key to treatment success and patient compliance should be confirmed prior to surgery. There currently is no consensus on indications for surgical treatment, as the relative risks and benefits of surgical and nonsurgical treatment continue to be debated.
If surgical treatment is elected, all of the pathologic changes of camptodactyly should be considered of these can be detected via clinical exam.
The initial skin incision should consider a potential volar soft tissue deficit following correction. A midline longitudinal approach with subsequent Z-plasties at closure can provide moderate length [6, 7] in most cases. Other approaches include zigzag Bruner incisions [3] or a large proximally based flap [3] that can incorporate a full thickness skin graft at closure if needed. A proximally based lateral finger transposition flap has also been proposed [16].
The incision extends from beyond the PIP joint to the proximal palm so that the distal edge of the transverse carpal ligament can be exposed. The FDS should be inspected from the carpal canal through its course under the A1 pulley. Proximal traction with the wrist in a neutral position should have the spring-like feel of a muscle belly. Lack of excursion may suggest a proximal fibrous origin. Distal traction should produce normal PIP flexion. Further exploration using windows in the annular ligaments may be necessary to find abnormal distal insertions.
The FDP should be identified and the lumbrical should be followed from origin to insertion. The lumbrical may insert abnormally into the MCP joint capsule, the FDS tendon, or the adjacent finger extensor system. If the lumbrical is found to have an abnormal insertion it can be released and re-inserted into the extensor apparatus.
Resection of the FDS can result in significant loss of finger flexion. Unless the origin is abnormal, the FDS can be used in a tendon transfer. If the preoperative exam demonstrates that PIP extension can be restored with stabilization of the MCP in flexion, the FDS can be transferred to the A1 pulley as a “lasso” to produce MCP flexion (Fig. 16.2a). If the preoperative exam demonstrates that PIP extension cannot be restored with stabilization of the MCP in flexion, the FDS is transferred to the extensor system so that it can act as both an MCP flexor and PIP extensor (see Fig. 16.2b).
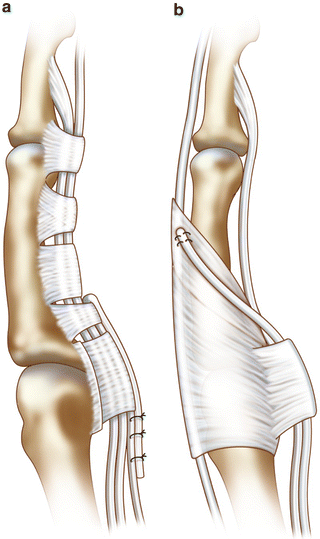

Fig. 16.2
(a) Zancolli lasso procedure. The FDS tendon is released from its insertion and sutured around the A2 pulley to act primarily as an MCP flexor. (b) Intrinsic transfer. The FDS tendon is re-routed to insert into the extensor apparatus to act as an MCP flexor and a PIP extensor
If the FDS has been found to act independently, it can be transferred without further dissection. If it is not independent, the FDS needs to be dissected and freed from the adjacent FDS. Alternatives for tendon transfer include an adjacent FDS [8] tendon or the extensor indicis proprius (EIP) re-routed volar to the intermetacarpal ligament [2, 17]. Gupta suggests that EIP transfer is only appropriate when stabilization of the MCP to prevent hyperextension allows full or near full active PIP extension.
When passive PIP extension cannot be restored after releasing the FDS insertion or anomalous intrinsic muscles, some authors perform releases of the volar plate, capsule, and/or ligaments. Any surgical release of the PIP joint results in considerable inflammation and scar and is likely an important factor in the loss of flexion noted in some series or case reports [4–7, 18, 19]. Siegert et al. reported poor results of surgical treatment [18] and McFarlane reported an average flexion to distal crease of palm of 1.8 cm [7]. In the latter series only 33 % of patients retained full flexion. McFarlane suggests that it is better to accept an incomplete correction of the flexion contracture rather than risking loss of flexion due to scarring about the PIP joint [6].
The results of surgery are variable and difficult to compare due to differences in methodology and incompatible formats of results reporting. A passively correctable PIP joint prior to surgery is generally favorable. In case reports where the PIP joint was not released there was no loss of flexion [12, 13, 17]. In case series where the PIP joint was rarely released, the loss of flexion was minimal and often less than 10° [4, 5]. Smith reported an improvement of 57° with surgery [5] and Foucher reported 68–88 % improvement depending upon the preoperative type [4].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







