10 Breast cancer
Diagnosis, therapy, and oncoplastic techniques
Synopsis
 Breast cancer afflicts one in eight women; screening mammography has been shown to reduce mortality from breast cancer by early detection of disease. Core needle biopsy is the preferred diagnostic strategy.
Breast cancer afflicts one in eight women; screening mammography has been shown to reduce mortality from breast cancer by early detection of disease. Core needle biopsy is the preferred diagnostic strategy.
 Breast cancer is a heterogeneous disease; several unique molecular subtypes of breast cancer have been identified based on the prognostic markers estrogen receptor, progesterone receptor and the HER2/neu oncoprotein.
Breast cancer is a heterogeneous disease; several unique molecular subtypes of breast cancer have been identified based on the prognostic markers estrogen receptor, progesterone receptor and the HER2/neu oncoprotein.
 Breast conserving surgery plus radiation provides equivalent overall survival to a total mastectomy, if feasible based on extent of disease.
Breast conserving surgery plus radiation provides equivalent overall survival to a total mastectomy, if feasible based on extent of disease.
 Decision making for adjuvant chemotherapy and radiation therapy involves tumor and patient specific features. These must be considered preoperatively by the surgical team in order to achieve optimal results.
Decision making for adjuvant chemotherapy and radiation therapy involves tumor and patient specific features. These must be considered preoperatively by the surgical team in order to achieve optimal results.
 Many stage 0, stage I, or stage II breast cancer patients planning for mastectomy and most patients electing for prophylactic mastectomy are candidates for immediate breast reconstruction.
Many stage 0, stage I, or stage II breast cancer patients planning for mastectomy and most patients electing for prophylactic mastectomy are candidates for immediate breast reconstruction.
Introduction
In the United States, breast cancer is the most common noncutaneous malignancy among women.1 The lifetime incidence of breast cancer for American women is approximately 1 in 8. Male breast cancer represents 1% of all breast cancers. On occasion, a patient will present to the plastic surgeon with breast complaints as well; thus, knowledge of the current diagnostic and therapeutic strategies is essential. The expertise of a plastic surgeon may be called upon regarding screening or the diagnostic evaluation of a previously augmented patient with breast complaints or abnormal imaging. Many patients opt for immediate or delayed reconstruction after mastectomy for breast cancer. It is crucial for plastic surgeons to understand the implications of adjuvant oncologic therapies on the suitability for immediate breast reconstruction. Plastic surgeons are an important part of the multidisciplinary care of the patient with breast cancer.
History
Independent risk factors for breast cancer include early age at menarche, late menopause, first full-term pregnancy after the age of 30 years, history of premenopausal breast cancer for a patient’s mother and sister (i.e., two or more 1st-degree relatives with a diagnosis of breast cancer at an early age), and personal history of breast cancer or of benign proliferative disease. The BRCA1 and BRCA2 mutations occur in <1% of women.2 These inherited mutations account for about 5% of breast cancers; thus, the majority of diagnoses are sporadic.3
Basic science
Several unique molecular subtypes of breast cancer have been described by gene expression microarray classification that have implications on prognosis, such as overall survival and distant metastasis recurrence free survival.4 These molecular profiles provide information in addition to the traditional histopathologic markers estrogen receptor (ER), progesterone receptor (PR), and the HER2/neu oncoprotein. These include the basal subtype (ER, PR, HER2/neu negative, or the so-called triple negative subtype, typically with high grade and high proliferation rates), the luminal A subtype (ER positive, PR positive, HER2 negative, typically with low expression of proliferation related genes) and the luminal B subtype (ER and PR positive but lower expression than luminal A; variable expression of HER2/neu, and high expression of proliferation related genes). While most triple negative tumors are basal-like by microarray, and most basal-like tumors are triple negative, discordance does exist about 30% of the time, which must be kept in mind when comparing studies with different classification strategies.5 Basal-like cancers are aggressive and share features with tumors arising from BRCA 1 mutation carriers. Triple negative cancers will not benefit from treatment with available targeted therapies, such as endocrine therapy for ER positive disease6 or trastuzumab, a monoclonal antibody directed at the HER2/neu oncoprotein receptor, for HER2 positive disease.7 Therefore, triple negative breast cancer patients generally are recommended cytotoxic chemotherapy, based on evidence-based guidelines.8
Gene expression predictors such as the Oncotype DX 21 gene recurrence score (Genomic Health, Redwood City, CA) and the Mammaprint 70 gene profile (Agendia, Irvine, CA) are being used increasingly to risk stratify patients regarding the need for adjuvant chemotherapy based on predicted clinical outcomes by primary tumor characteristics.9,10 These molecular assays are gaining acceptance by the oncology community as decision making tools. The Oncotype DX 21 gene recurrence score has been incorporated into the NCCN guidelines for breast cancer management.8 The American Society of Clinical Oncology (ASCO) has published guidelines for use of tumor markers in the management of breast cancer.11
In recent years, the breast cancer research community has focused much attention on the stem cell theory, which contends that only a small fraction of breast parenchymal cells possess the inherent ability to self-renew, differentiate or cause tumorigenesis.12 The stem cell theory is in contrast to the traditional clonal evolution, multi-hit theory.13 Several investigators have made important contributions in recent years in the study of putative breast cancer stem cells. Al-Hajj et al. reported the ability to identify tumorigenic stem cells based on surface markers (CD44 positive, CD24 negative) and showed that as few as 100 of such cells form tumors in immunocompromised mice, whereas tens of thousands of unselected cells typically fail to form tumors.14 Dontu et al. developed a suspension cell culture technique that allows for selection of tumorigenic stem cells from tumor specimens.15 Shackleton et al. demonstrated the ability of a single breast stem cell to generate a functional mammary gland.16 Understanding the biology of breast stem cells may provide an opportunity to target this refractory population of slow growing stem cells, which are resistant to standard cytotoxic chemotherapy and radiotherapy.
Patients who carry a deleterious BRCA 1 or BRCA 2 mutation have an elevated risk of breast cancer diagnosis – about a 60–80% lifetime risk.2 Hundreds of known mutations exist and may be detected via a blood test for genetic sequencing. BRCA 1 and BRCA 2 are important for DNA double strand break repair.17 These patients also have elevated risk of contralateral breast cancers, ovarian cancer, fallopian tube cancer and other types of malignancy due to their inability to repair damaged DNA. For this reason, clinicians are increasingly referring patients to genetic counselors for consideration for genetic testing. Stage for stage, BRCA mutation carriers have equivalent prognosis to non-BRCA carriers, however, BRCA 1 related tumors tend to be of the aggressive basal-like subtype and tend to afflict younger women.
Diagnosis
Diagnostic imaging modalities
Mammography
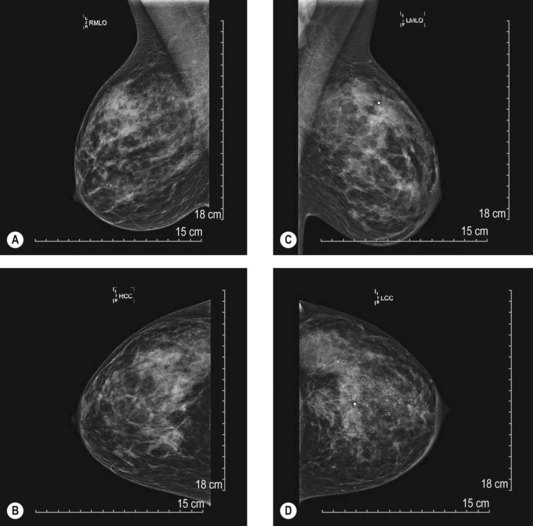
Mammography is a highly effective screening modality. The typical views include a mediolateral oblique view and a craniocaudal view. With questionable lesions, specific magnified views are obtained to better delineate the area of interest. The American Cancer Society (ACS) guidelines recommend beginning screening mammography at age 40 and continuing annually, as long as they are in good health. Although recently recommendations made by the US Preventive Services Task Force based on a systematic review of the literature prompted much controversy and debate,18 clinically the ACS guidelines continue to be usual practice.19
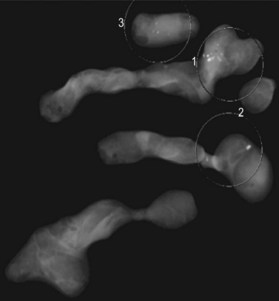
The BIRADS (Breast Imaging Reporting and Data System) classification (Table 10.1) is the most commonly used reporting system for mammography.20 These standardized criteria provide a means of radiographically risk-stratifying lesions such that the significance of the mammogram is clear to all clinicians. In this classification, BIRADS 4 (suggestive of malignant disease) or BIRADS 5 (highly suggestive) should always prompt biopsy for tissue diagnosis of a mammographic abnormality (Fig. 10.1). The more frequent use of mammography for screening purposes has resulted in increased recommendations for breast biopsies for clinically occult lesions. As a result of screening mammography, many breast cancers may now be detected at an earlier stage; it is estimated that implementation of regular mammography for women older than 50 years can reduce breast cancer mortality significantly for this population.21
Table 10.1 The BIRADS classification is used to classify screening and diagnostic mammograms, breast ultrasounds and breast MRIs; all breast imaging receives a score based on the BIRADS classification, which helps determine if further evaluation and possible biopsy are indicated
| BIRADSa category | Interpretation |
|---|---|
| 0 | Assessment incomplete; needs additional imaging evaluation |
| 1 | Negative; routine screening mammogram indicated in 1 year |
| 2 | Benign finding; routine screening mammogram indicated in 1 year |
| 3 | Probably benign finding; short interval follow-up study (usually 6 months) indicated |
| 4 | Suspicious for malignancy; core biopsy recommended at this time |
| 5 | Highly suspicious for malignancy; core biopsy recommended at this time |
| 6 | Known biopsy proven breast cancer |
a Breast Imaging Reporting and Data System, developed by The American College of Radiology.
(Reproduced from Balleyguier C, Ayadi S, Van Nguyen K, et al. BIRADS classification in mammography. Eur J Radiol. 2007;61(2):192–194.)
Ultrasonography
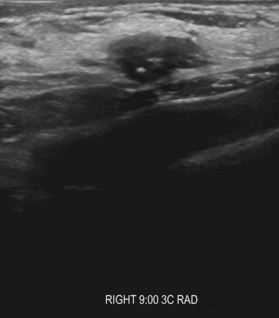
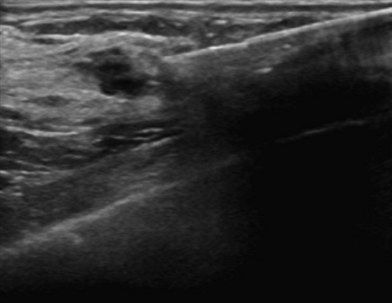
Breast ultrasonography is widely used as a diagnostic modality that typically plays a role in the work-up of all breast masses; moreover, ultrasound interpretations also utilize the BIRADS classification to describe radiographic findings (Fig. 10.2). Other indications include evaluation of palpable masses in young women with radiographically dense breasts, evaluation of masses in conjunction with diagnostic mammography, and guidance for biopsy or evacuation of cysts.22 Many breast surgeons use focused breast ultrasonography as an extension of the physical examination. Several ultrasonographic criteria help differentiate malignant from benign lesions, including the presence of spiculations, a taller than wide shape, multiple the presence of calcifications, angular margins, a markedly hypoechoic lesion, posterior shadowing, and a heterogeneous internal structure.23,24
Magnetic resonance imaging
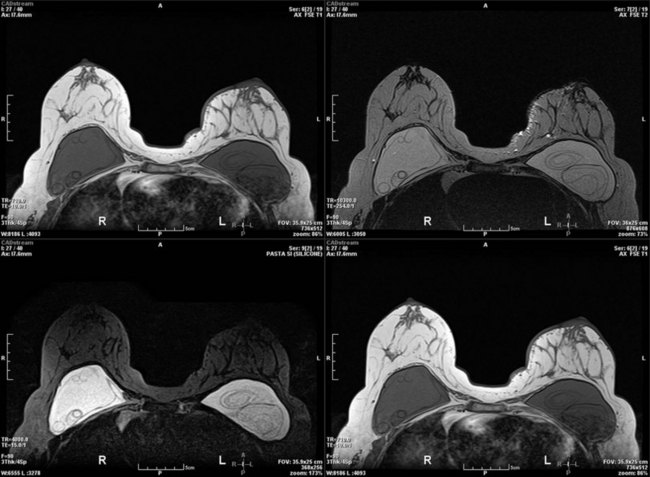
In recent years, contrast-enhanced magnetic resonance imaging (MRI) has been used for the evaluation of the breast for cancer. One controversial study found that as many as 10% of newly diagnosed breast cancer patients may have a contralateral primary tumor.25 A prospective randomized controlled trial found no difference in reoperation rate for newly diagnosed breast cancer patients based on whether or not MRI was ordered as part of the diagnostic work-up.26 A recent consensus conference proposed guidelines for the use of MRI as a diagnostic and screening tool.27 MRI is extremely helpful for screening patients at high risk for breast cancer, such as individuals who carry a deleterious BRCA mutation. MRI is also useful in the management of patients with axillary node metastases and unknown primary. The main disadvantages are cost (which precludes widespread use as a screening modality), issues with specificity leading to additional needle biopsies and limited availability compared with mammography or ultrasonography. Several centers have reported increasing mastectomy rates correlating with increasing use of breast MRI.28 MRI is best reserved as a problem solving tool for specific indications in the diagnostic work-up of breast cancer and may not be necessary for all breast cancer patients. However, the United States Food and Drug Administration recommends monitoring patients with silicone breast implants for occult rupture with breast MRI performed 3 years postoperatively, then every 2 years thereafter. The “linguine sign” indicates evidence of ruptured silicone breast implants on MRI (Fig. 10.3).
Diagnostic imaging in patients with breast implants
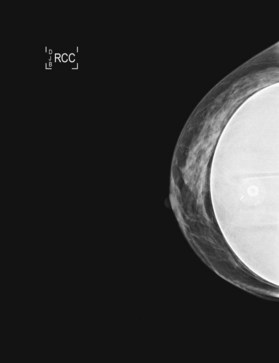
As implants are widely used in both reconstruction and augmentation of the breast, the efficacy of breast cancer screening with mammography is important in this population. A breast implant is radiopaque on mammography and obscures some breast tissue. This, seemingly, may reduce visualization and therefore compromise detection of breast tumors. Efforts to limit the impact of implants on imaging of the breast include submuscular placement of the device – theoretically allowing better definition and separation from overlying breast tissue – as well as special mammographic techniques. These mammographic approaches include additional surveillance views of the augmented breast tissue and displacement of the implant away from the breast tissue.29 Mobility of the implant is critical to the efficacy of these techniques. If the implant is not freely mobile, a straight lateral view is obtained in an attempt to more adequately visualize the tissue posterior to the implant. Factors that restrict the mobility of the implant – such as an overly large implant in proportion to the overlying breast tissue, or an implant that has a calcified or contracted and rigid peri-prosthetic capsule – can significantly impair the efficacy of mammography in terms of adequate visualization, particularly the posterior aspect of the breast.
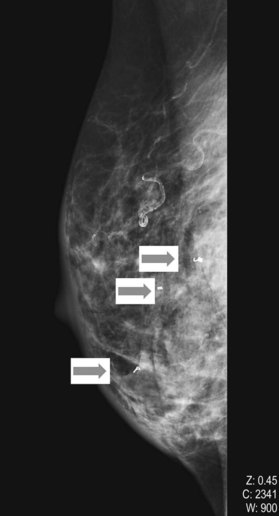
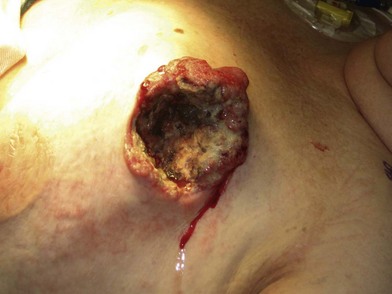
The estimated proportion of breast tissue that is obscured from mammographic examination ranges in the literature from 25% to 80%.30 However, stage at presentation for augmented patients was no different from that for the patients without implants in two retrospective analyses.31,32 Holmich et al. reported a study of 2955 patients who underwent breast augmentation between 1973–1997;33 the authors found that women with breast implants on average were diagnosed with breast cancer at the same stage as were control subjects. There was no significant difference in overall survival between the two groups after an average of 6.4 years of follow-up. Several studies have reported that augmented women do not have a higher incidence of breast cancer.34–36 There has been supposition that patients with breast implants have an enhanced sense of body image and self with a greater percentage of self-reported breast cancers and attention to examination, although these data are somewhat empiric in nature. Recently, Xie et al. reported a comparison of a cohort of 24 558 women who underwent bilateral cosmetic breast implants to a cohort of 15 893 women who underwent other types of plastic surgical procedures between 1974–1989.37 In the largest series to date, the authors reported that women who had undergone prior augmentation mammoplasty were more likely to present with more advanced stage breast carcinoma. This may suggest, in contradistinction to prior study, that breast implants delay detection.37 Additionally, diagnostic biopsies such as core needle or excisional biopsy may risk rupture of the implant (Fig. 10.4).
Histologic diagnostic modalities
The majority of lesions for which women undergo breast biopsy prove to be benign. In addition, many women must undergo multiple biopsies over the course of their lifetime. No breast biopsy technique has 100% accuracy, however, large gauge image guided core needle biopsy closely approximates results of open surgical excision of breast lesions.38,39 The most widely used techniques for breast biopsy is percutaneous image guided core needle biopsy.27 Core biopsy is advantageous in terms of minimal scar, decreased anatomic deformity permitting improved mammographic surveillance (minimal or no scarring on subsequent mammograms), decreased pain, and significantly decreased cost while diagnostic accuracy is maintained. The diagnosis of cancer is thus generally known preoperatively, facilitating appropriate planning and counseling about treatment options. Furthermore, diagnosis by core biopsy allows for proper surgical planning, including the possibility of a nodal evaluation procedure (if indicated) as well as appropriate planning for resection with adequate margins.
Image-guided core biopsy
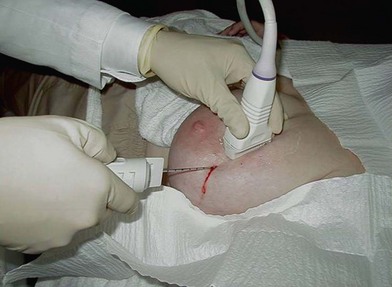
Image-guided core biopsy is performed by either stereotactic (Fig. 10.5) or ultrasound guidance (Fig. 10.6). Stereotactic biopsy is indicated when planning breast biopsy of lesions only visible mammographically (i.e., calcifications). Ultrasound guidance is increasingly being incorporated into surgical practice in the care of breast disease; it is indicated when planning breast biopsy of lesions well visualized sonographically. Ultrasonography provides excellent guidance for core biopsy, fine-needle aspiration, and drainage of cysts.
Fine-needle aspiration
Fine-needle aspiration (FNA) is performed under guidance by palpation, ultrasonography, or stereotactic mammography. The examiner places a 20- to 23-gauge needle into the lesion. On aspiration with a syringe, material is sampled with multidirectional passes for cytopathologic examination. The cellular material is placed on slides for review by a cytopathologist. FNA typically does not provide enough tissue architecture for the diagnosis of invasive carcinoma to be made.40 When atypia is found on FNA, 61% ultimately proves to actually be malignant disease.41 Therefore, findings of atypia should result in surgical excision. The biggest pitfall to use of FNA is the problem of sampling error; false-negative rates of 2–22% have been reported in the literature.42,43 FNA provides cytologic diagnoses that must be closely correlated with clinical and imaging findings to improve sensitivity of diagnosis (the so-called triple test of clinical, radiographic, and pathologic findings). In many centers, core needle biopsy has supplanted FNA as the diagnostic breast biopsy of choice due to high accuracy, accurate histopathology with prognostic markers while remaining minimally invasive.
Core needle biopsy
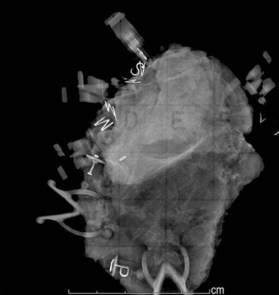
Core needle biopsy involves use of a biopsy device with an 8–14-gauge needle (Fig. 10.7). The needle is positioned by either stereotactic or sonographic guidance (Fig. 10.8).38 Multiple passes are required to optimize sensitivity and to determine histologic grade with accuracy.44 Often, modern core biopsy devices use vacuum to aspirate tissue into a chamber, where it is sampled. With suction assistance, high-speed oscillating knives pull a cylinder of tissue into a chamber outside the breast. The advantage of these techniques is that multiple passes are accomplished by rotating the needle sequentially rather than by reinsertion; thus, only one needle stick is required. Accuracy of the vacuum-assisted breast biopsy approaches 100%.38,45 Core biopsy specimen provides enough tissue to analyze estrogen receptor status and the presence of the HER-2/neu oncoprotein, neither of which is normally ascertainable by an FNA specimen. A titanium tissue marker clip is inserted to mark the site of the core needle biopsy; multiple types of clips exist to help demarcate biopsies of different lesions (Fig. 10.9).
Excisional biopsy
With the increasing use of percutaneous image-guided biopsy, indications for excisional biopsy have evolved to include disagreement between the mammographic or clinical impression and histologic findings of percutaneous biopsy; atypical ductal hyperplasia on percutaneous biopsy46,47; and radial scar on mammography or percutaneous biopsy.48 When percutaneous biopsy is nondiagnostic for nonpalpable radiographic lesions, a needle-localized breast biopsy is recommended. It is important to emphasize that clinical judgment must determine decision-making whether or not to excise radiographically occult breast masses based on clinical grounds. A nonsuspicious fibroadenoma of the breast diagnosed by ultrasound or core biopsy may be observed with serial follow-up; reasons to consider excisional biopsy for a fibroadenomas include patient preference, suspicion of phyllodes tumor, or a growing or painful lesion.
Patient selection
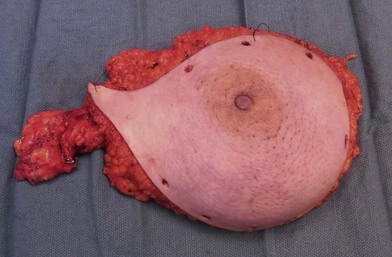
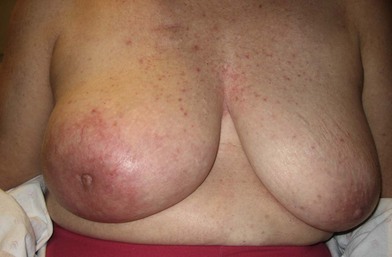
A breast cancer patient’s decision making regarding choice of breast surgical procedure is influenced by numerous factors, including ratio of breast size to tumor burden, stage, multicentricity of disease, family history, age, availability of radiation therapy, personal preferences, and biases by her providers. The multidisciplinary team caring for breast cancer patients includes breast surgical oncologists, medical oncologists, radiation oncologists, radiologists, pathologists, nuclear medicine specialists and plastic surgeons – each with their own meaningful role in the management of breast cancer. Decision making regarding indications for adjuvant chemotherapy and radiation therapy is an important topic but beyond the scope of this chapter; a detailed summary of standard of care evidence based algorithms may be found published in the NCCN guidelines.8 Patients diagnosed initially with clinically Stage III breast cancers are evaluated with a staging work-up to rule out metastasis prior to initiating systemic therapy or surgical resection (Fig. 10.10). Clinicians and patients may consider neoadjuvant or preoperative systemic therapy (chemotherapy or endocrine therapy) to reduce the tumor burden such that a lumpectomy may be feasible. A neoadjuvant approach also permits determining if a patient responded to the chosen therapy, which provides valuable prognostic information (examples of locally advanced and inflammatory breast cancers are shown in Figures 10.11–10.14). In general, there is no survival advantage for a neoadjuvant approach versus surgery first, unless the patient has a pathologic complete response which implies a more favorable prognosis.49
Treatment/surgical technique
Breast conserving surgery
The management of early-stage breast cancer with a partial mastectomy – lumpectomy, quandrantectomy, or segmentectomy – followed by adjuvant radiation therapy is collectively termed “breast conserving therapy” or “breast conserving surgery.” This approach is appealing to a number of women. Segmental mastectomy, along with radiation, has been shown to be roughly equivalent to mastectomy in long-term survival in a number of prospective trials. These randomized studies with follow-up periods from 6 to 13 years found no difference in overall or distant disease-free survival between lumpectomy versus mastectomy.50–52 In the landmark National Surgical Adjuvant Breast/Bowel Project (NSABP) B-06 trial, local recurrence rates for lumpectomy with adjuvant therapy were 14.3%; with lumpectomy without radiation therapy local recurrence rates were 39.2%. Accordingly, these data punctuate the need for adjuvant radiation for successful treatment with partial mastectomy in the majority of patients.52 The NSABP results may be limited by the definition of margins used in the study. In this report, negative margins were defined as “no tumor on ink,” while most surgeons prefer a wider free margin (typically ≥0.2 cm). As several of the studies suggesting a higher recurrence rate with breast conserving approaches did not have well-established negative margin criteria, some of the early reports of higher local-regional recurrence with breast conserving therapies are subject to interpretation.
Breast cancer recurrence is complex and multifactorial. Factors that contribute to recurrence after breast conserving therapy, such as young age, no systemic therapy, aggressive disease, etc., also predispose the patient to recurrence after mastectomy. Recurrence relates not only to the operative technique, but also to intrinsic tumor characteristics as well as adjuvant and the neoadjuvant therapies utilized. Modern local recurrence rates may be inferred from the Early Breast Cancer Trialists’ Collaborative Group overview which found 5-year local recurrence rates of 7% for lumpectomy with radiotherapy compared with 26% for lumpectomy without radiotherapy.53 This large overview of 78 prospective randomized trials of early breast cancer found that if four breast cancer recurrences could be prevented, one breast cancer related death would be averted.53
Technique
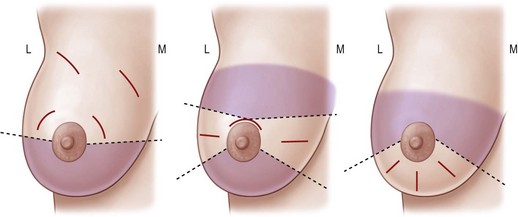
The diagnosis is confirmed by a core biopsy preoperatively, and the patient is re-examined before the operation is commences. The lesion is marked while the patient is awake to facilitate location of the tumor in the operating room. If the lesion is not palpable then wire localization or ultrasound may be used to identify the location of the lesion or biopsy clip. Incision selection is demonstrated in Figure 10.15.
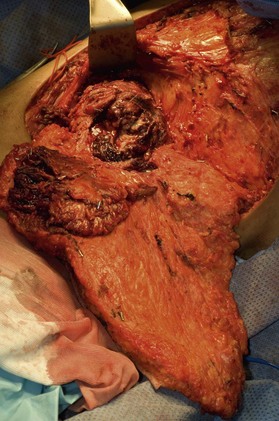
After the incision is made, tissue forceps are used to grasp the skin and electrocautery is used to dissect circumferentially around the tumor including a margin of grossly normal tissue. The dissection may continue to the pectoralis fascia for the deep margin, but for small, superficial tumors, this depth of resection may not be necessary. If the tumor is near the skin, an ellipse of skin should be resected to ensure negative margins. The tumor is oriented with marking sutures. The resection specimen is sent for an ex vivo radiograph to ascertain whether the calcifications in question were removed. Breast conserving surgery carries with in an inherent risk of close or positive margins microscopically on permanent section histopathology (range 9–32%).54–57
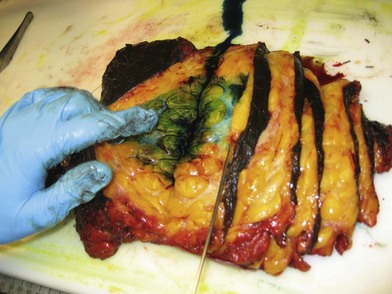
Meticulous intraoperative margin assessment must be followed to ensure the best possible outcome in breast conserving procedures. Intraoperative processing of the partial mastectomy is extremely important; needle localization with radiographs (Fig. 10.16) and stepwise sectioning (Fig. 10.17) have been utilized to decrease the rate of positive margins. This necessitates a team of radiologists and pathologists to ensure that the safest minimal volume of tissue adjacent the tumor is resected – thus enhancing the aesthetic outcome – while optimizing the likelihood that adequate and accurate margins are obtained. While the extensive intraoperative process is time consuming, it can significantly decrease the need for re-excision to achieve negative margins and enhance the aesthetic results.58 If there is any concern about margins based on surgical, radiographic or pathology input, the lumpectomy cavity is re-excised prior to closure. These re-excision specimens are sent separately from the main specimen for pathologic evaluation. If there are questions regarding margin status, it may be best to delay the plans for reconstruction in conjunction with the conserving surgery to avoid any transposition of local tissues that might confound re-excision.