Breast Brachytherapy Device Placement
Peter D. Beitsch
Essentially all patients undergoing breast conservation therapy should have adjuvant radiation therapy. Traditionally, this has been whole breast irradiation, but over the last 15 years some patients have had only the perilumpectomy portion of their breast treated with radiation given over shortened periods of time—accelerated partial breast irradiation (APBI). Not all lumpectomy patients are candidates for APBI, but general recommendations have been put forth by both the American Society of Breast Surgeons and the American Brachytherapy Society (Table 16.1). Patients falling outside of these parameters can be treated on an individualized basis—always in consultation with the radiation oncologist.
Individual Brachytherapy Devices
Balloon Devices
MammoSite Balloon
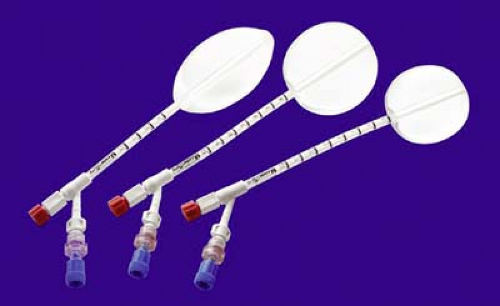
The first generation of MammoSite balloon was approved by the U.S. Food and Drug Administration in May 2002. The balloon is made of silicone and has a single treatment lumen. It comes in two spherical sizes—4 to 5 cm and 5 to 6 cm—as well as a 5 cm elliptical size (Fig. 16.1). The main advantages of the MammoSite balloon are the comfort of the soft silicone and the familiarity of the device (it has been available the longest of any of the brachytherapy devices). The main disadvantages are the need for a minimum of 5 mm of skin-to-cavity distance (preferably >7 mm) and the soft balloon may not expand symmetrically, causing the central treatment lumen to be offset and therefore unusable. A second-generation device made of polyurethane is now available that overcomes the symmetry issue but not the skin spacing problem. A third-generation device with multiple catheters is available. The MammoSite ML has a central catheter and three catheters surrounding it. These multiple catheters allow the treatment plan to more precisely tailor the prescription dose to the cavity avoiding excess dose to the skin and chest wall. The balloon is filled with a combination of saline and a small amount of contrast such as Isovue.
Table 16.1 Patient Selection Criteria | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Contura
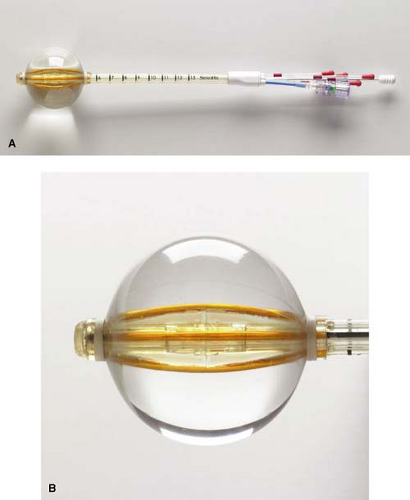
SenoRx has developed a single-insertion device called the Contura with multicatheters within a balloon (Fig. 16.2). It comes in two sizes—a 4- to 5-cm diameter balloon and a 5- to 6-cm diameter balloon. It looks very similar to the MammoSite with similar insertion methods; however, the multiple catheters allow the treatment plan to more precisely tailor the dose to the cavity, avoiding normal structures such as heart, lung, rib, and skin (thus allowing a narrower skin-to-cavity distance). In addition, the device has suction parts at each end of the balloon to aspirate fluid and air from the cavity. The main disadvantage of Contura is the stiffness of the catheters, which can be uncomfortable to some patients. The balloon is filled with a combination of saline and a small amount of contrast such as Isovue.
By the end of 2009, approximately 6000 devices will have been placed and only short-term data are available for this device. The company has begun a registry trial with a targeted accrual of approximately 400 patients.
Multicatheter Bundled Devices
SAVI
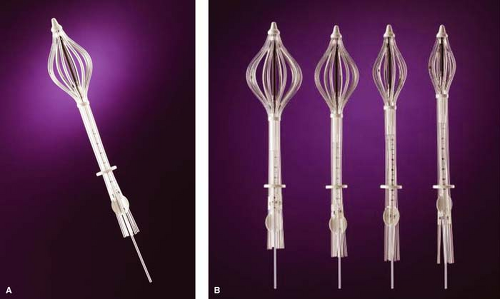
Cianna has developed a single-insertion, multicatheter device called the SAVI (Fig. 16.3). It comes in 6-mini, 6, 8, or 10 catheters depending on the size of the lumpectomy cavity. The catheters are in direct contact with the lumpectomy walls. SAVI can be used in almost all cavities but is particularly suited for small (<30 cc) cavities (6-mini) and long, elliptical cavities. The multiple catheters allow the treatment plan to more precisely tailor the dose to the cavity, avoiding normal structures such as heart, lung, rib, and skin (thus allowing a narrower skin-to-cavity distance). Although there is a newer version of SAVI with more flexible catheters now available, the main disadvantage of the device
is the stiffness of the catheters, which can be uncomfortable to some patients. By the end of 2009, approximately 3000 patients will have been treated with this device and only short-term treatment data are available.
is the stiffness of the catheters, which can be uncomfortable to some patients. By the end of 2009, approximately 3000 patients will have been treated with this device and only short-term treatment data are available.
New Brachytherapy Sources
Xoft
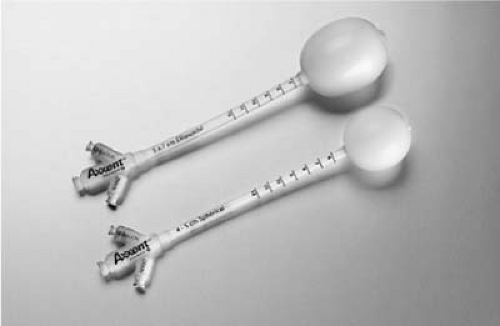
Xoft has developed a miniaturized X-ray radiation source, the Axxent system, that replaces the radioactive iridium seed and is delivered through their own single-catheter balloon devices (Fig. 16.4). The “source” is emitting radiation only when turned on and is of a much lower energy. This low-energy “source” allows the patient to be treated outside the radiation vault in places such as physician offices. The shielding required is similar to that needed for routine office X-rays. They have their own set of balloon applicators that have a barium-impregnated balloon and are inserted similarly to the MammoSite catheter. By the end of 2009, more than 500 devices had been used for treatment.
The EXIBT (Electronic Xoft Intersocietal Brachytherapy Trial) registry under the oversight of the American Brachytherapy Society, American Society of Breast Surgeons, and American College of Radiation Oncology has accrued 69 patients as of the end of 2009. Extensive treatment parameters (V150, V200, V300, etc.) have been collected along with short term/long term complication data and recurrence/survival data.
Zeiss
Zeiss has also developed a low-energy electronic radiation source that currently is used only intraoperatively; however, there are plans underway to develop balloon applicators for use in minimally shielded environments similar to the Xoft Axxent system. The TARGIT randomized study of intra-operative single dose radiation versus traditional whole breast irradiation has completed accrual. The results will be published in the near future.
Brachytherapy devices are usually placed postoperatively in the physician’s procedure room. This allows the final pathology to be evaluated to ensure the patient is a good candidate for APBI. In addition, at least for the MammoSite balloon catheter, there may be a lower short-term infection rate and lower long-term seroma rate with postoperative placement.
Postoperative placement begins with the preoperative recognition that a patient is a potential APBI candidate. Once identified, APBI can be incorporated into the overall treatment discussion with the patient as a possibility for her postoperative adjuvant radiation. This is also a good time to have the patient consult with the radiation oncologist. This interdisciplinary interaction is crucial for successful APBI treatment.
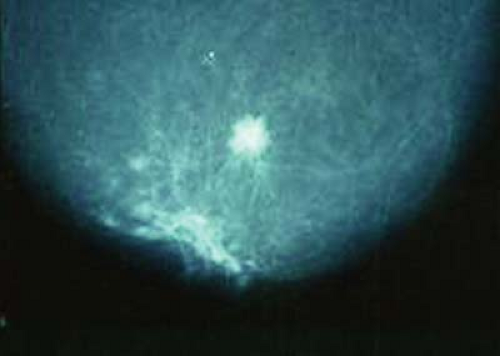
Occasionally, preoperative workup includes breast magnetic resonance imaging to ensure there is no multicentric disease or the tumor is not larger than suspected. However, most APBI candidates are postmenopausal women with involuted breasts and fat density; mammograms show their cancers clearly (Fig. 16.5).
Pertinent Anatomy
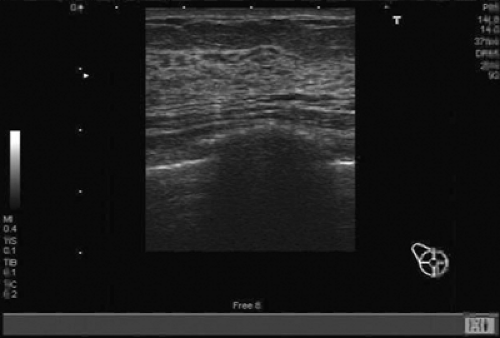
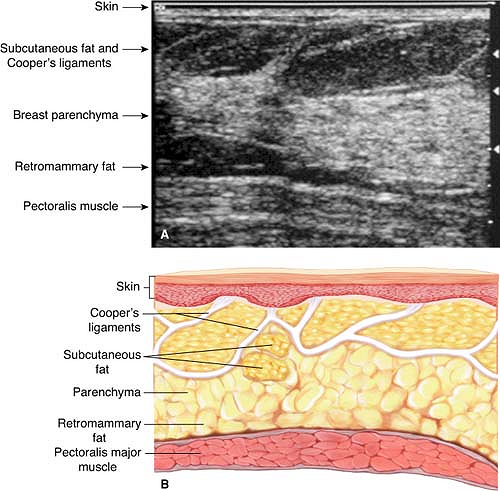
The placement of a breast brachytherapy device requires a basic understanding of breast anatomy as well as a minimum level of ultrasound knowledge of the breast (Figs. 16.6 and 16.7). Ultrasound assessment includes skin-to-cavity distance, general gestalt of the shape, and actual dimensions of the cavity (height, width, and length) (Figs. 16.8 and 16.9). This evaluation of the cavity will aid in the choice of brachytherapy device as well as determining the size of the device needed.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree