CHAPTER 71 Botox® for face, neck and brow
History
Clostridium botulinum bacterium was first recognized and isolated in 1896 by Emile van Ermengem. In 1944, Edward Schantz successfully cultured Clostridium botulinum and was able to isolate the potent toxin. In 1949, Burgen’s group discovered that botulinum toxin blocks neuromuscular transmission. C. botulinum bacteria produce eight serologically distinct types of botulinum neurotoxins designated as type A, B, C1, C2, D, E, F and G. Type A seems to be the most potent in humans. During the 1950s, Dr Vernon Brooks started experimenting with botulinum toxins for medical purposes. His work was later significantly advanced by the works of Dr Alan Scott during the 1970s. In 1977, botulinum neurotoxins were used in humans for the first time. Botulinum toxin type A (BTX-A) was granted limited approval by the FDA for the treatment of strabismus in 1979. The approval was later extended to blepharospasm in 1985. The FDA approved the use of Botox® botulinum toxin type A (Allergan Inc., Irvine, CA USA) for these two conditions and for the treatment of hemifacial spasm in 1989. On April 15 2002 Botox® Cosmetic was granted FDA approval for the treatment of moderate to severe glabellar rhytides by extensive clinical trials following observations on the cosmetic effectiveness on glabellar frown lines by Drs Jean and Alastair Carruthers made in 1987 while treating a patient with blepharospasm.1
As a commercially available unique pharmaceutical agent, Botox® popularity continues to grow and expand into other therapeutic applications as well as cosmetic improvement of a wide range of facial wrinkles in addition to those for which it was initially approved.2 Since its discovery as a wrinkle reducer, Botox® is the leading male and female minimally-invasive cosmetic procedure in the U.S. with more than 4.6 million treatments performed in 2007 alone, a remarkable 488% increase since the year 2000.3
This neurotoxin acts by inhibiting the release of the neurotransmitter acetylcholine at the neuromuscular junction of striated muscle fibers. This inhibition produces selective chemical reversible denervation of the muscle and as a result muscle activity is temporarily diminished. The toxin does not damage the nerves or alter the production of acetylcholine.4 Botox® weakens muscle action in approximately 2 to 4 days following injection and reaches maximal muscle denervation between 7 to 10 days. The effect is temporary since the terminal nerve endings start to form “peripheral sprouts” with time.
Following the appropriate dose and injected on the target muscles, botulinum toxin can temporarily address some of these senescent changes of the facial skin. It is the experience of these authors that with repeated treatment some degree of muscle atrophy may occur; therefore, subsequent treatments might require fewer units of toxin at longer time intervals. However, resistance has been seen in patients treated for neurologic conditions where doses are in the 100–200 units (U) range5 and it is currently a concern that cosmetic patients might develop neutralizing antibodies with repeated treatment.
Physical evaluation
• Obtain a complete medical history.
• Obtain a complete list of concomitant medications.
• Evaluate for history of muscular disorders.
• Evaluate for any signs of skin infection or inflammation.
• Evaluate for any disorder affecting the eyes.
• Evaluate for unequal eyebrow height.
• Obtain list of past cosmetic procedures including fillers and Botox® history.
• Botox® treatment is contraindicated during pregnancy, breastfeeding or patients planning to become pregnant.
Technical steps
Treatment of the upper face
Botox® Cosmetic has been proven to be a safe and effective modality for treating wrinkles of the upper face. This area has provided the majority of clinical experiences with Botox®. Glabellar frown lines, horizontal forehead lines and crow’s feet are the most common manifestation of the aging upper face. With the success of Botox® treatment in the upper face, clinicians have been expanding this therapy into other areas of the face.6 However, Botox® is only approved by the Food and Drug Administration for the treatment of glabellar frown lines.
Glabellar frown lines
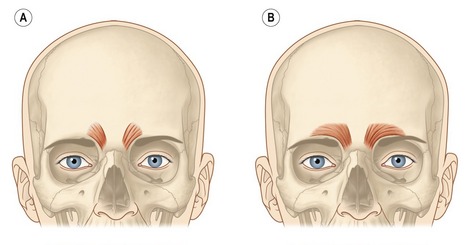
The underlying muscular activity of the procerus, corrugator supercilli and depressor supercilli muscles is responsible for the formation of the glabellar frown lines. The synergetic hyperactivity of these muscles produces the usually undesired facial appearance of age, fatigue and frustration. The procerus is a thin, narrow brow depressor muscle. Once contracted, it draws the medial aspect of the eyebrows down, producing transverse wrinkles over the nasal bridge. This muscle should be injected in the midline, slightly caudal to the root of the nose. The contraction of the corrugator supercilli muscle adducts and depresses the eyebrow, moving it inferiorly and medially. Repetitive contraction of the corrugator supercilli muscles produces the vertical glabellar creases. There are two well distinct patterns of this muscle. It can have a short, pyramidal shape located at the medial end of the supraorbital ridge (Fig. 71.1A) or a long and narrow shape extending along the supraorbital ridge reaching or even going beyond the mid-brow (Fig. 71.1B). Treatment of this muscle with Botox® should follow the respective anatomical pattern of the muscle. The depressor supercilli is a brow depressor and believed to be part of the orbicularis oculi muscle. The appropriate knowledge of the existence of this muscle allows for proper inactivation with Botox® injections. Several factors of the glabellar complex should be observed prior to treatment. Physical characteristics, such as brow arch, brow asymmetry, brow ptosis and muscle mass of the glabellar varies between male and females. Males tend to have a stronger muscle mass; therefore, higher doses in the range of 60 to 80 U of Botox® are often necessary to achieve the desired effect. Doses in the range of 30 to 40 U are usually sufficient to produce a satisfactory result on females. Injection techniques vary by clinicians. The following injection technique is used by the authors’ clinic:
• Following sterile technique and having the patient positioned with the chin down and head slightly lower that clinicians’, inject the procerus with 5–10 U mid-line and at a point below a line joining the brows.
• Next inject the center of the corrugator with 5 U above the caruncle of the inner canthus. Always inject above the bony supraorbital ridge. It is recommended that injectors maintain their non-dominant thumb on the orbital rim to avoid injection within the orbit.
• Then inject 5 U at 1 cm above the previous injection site and 3–5 U into a point 1 cm above the supraorbital rim in the mid-pupillary line. Repeat injections on the contralateral side to achieve a balanced outcome (Fig. 71.2).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree