Bites and Stings of Terrestrial and Aquatic Life: Introduction
|
Humans are a part of the environment and ideally live peacefully with other animals and plants. However, sometimes the interactions between man and the environment prove harmful to one or the other. The first two sections of this chapter consider the harmful effects of land-borne animal bites, as well as the bacterial and viral infections they may transmit. The last section reviews bites and stings and other forms of injury that may be inflicted by marine life.
Bites of Land Animals
Dog bites account for 80%–90% of all mammalian bites involving humans. Four to five million dog bites are estimated to occur each year in the United States, and, in 2001, it was estimated that 368,245 persons were treated for dog bite-related injuries.1 The human victim is often a 5– 9-year-old boy who may have been teasing or playing with the dog. Sometimes the bite occurs when a person is trying to break up a pair of fighting dogs or trying to aid an animal. In the United States 4%–5% of dog bites are work related and include bites sustained by postal carriers. Overseas, a higher percentage are produced by untamed animals.2–4 Most dog bite injuries involve the upper extremities, especially the hands. Cat bites are the second most common type of mammalian bite after dog bites, but the wounds following a cat’s bite are nearly twice as likely to become infected as wounds from dog bites.5
The evaluation and treatment of all bite wounds should include taking a careful history of the incident, the type of animal, the site of the bite, and the geographic setting. Hand wounds, puncture wounds, and crush injuries are likely to become infected. Specimens from infected bites should be cultured and a Gram-stained smear prepared; the wound should then be washed, well-irrigated, and left open. Most patients with bites on the hands, deep cat bites, deep cat scratches, and sutured wounds should be treated with amoxicillin/clavulanic acid or ceftriaxone because of the risk of Pasteurella multocida infection; if the patient is allergic to these agents a quinolone or a tetracycline should be used. Evidence that the use of prophylactic antibiotics is of benefit exists only for bites of the hand. However, once the patient has an infection, intravenous antibiotics and surgical drainage are often required.4,6–8 Tetanus immune status should be evaluated, and rabies prophylaxis should be considered, depending on the type of animal, local epidemiologic factors, and ability to quarantine the animal for 10 days.9
Human bites and monkey bites deserve special mention, because 30% become infected with aerobic or anaerobic mouth organisms. Infection caused by anaerobes may spread through the metacarpal–phalangeal space and causes severe damage. The same procedure should be followed as for other animal bites; that is, culture and Gram stain, thorough washing, and debridement. Wounds, especially hand wounds, should be left open if possible. Evidence suggests that patients with human bites that penetrate the epidermis should be treated prophylactically with amoxicillin/clavulanic acid for 7–10 days or, if the patient is allergic to penicillins, with a fluoroquinolone plus an antianaerobic agent such as clindamycin.7,10 Clenched-fist injuries should be evaluated by a hand surgeon because of the high likelihood of infectious complications, including septic arthritis and osteomyelitis.2
(See Chapter 183). An organism commonly infecting bite wounds is P. multocida, a bacterium present in the nasopharynx in 50%–66% of dogs and 70%–90% of cats. The most common pattern is that of local infection with adenitis after a dog or cat bite or scratch. P. multocida is the most common pathogen in cat bite infections and may also complicate 26% of dog bite wounds.5 The infection usually presents within 24–48 hours, often within several hours. In patients with a cat bite, the infection may be followed by tenosynovitis or osteomyelitis as a result of inoculation of the organism into the periosteum by the long, sharp tooth of the animal. Pasteurella infections have also been reported after bites of large cats such as lions and tigers.
Systemic infection may also occur as bacteria may enter the respiratory tract through the inspiration of contaminated barn dust, or by inhalation of infectious droplets sprayed by the sneeze of an animal. In such cases, the bacteria probably colonize the respiratory tract and in some patients cause active infection. Bronchiectasis, emphysema, peritonsillar abscess, and sinusitis have all been described with this organism. Finally, systemic infection with bacteremia or meningitis may occur.
P. multocida is a small, Gram-negative, ovoid bacillus that grows well on blood agar but does not grow on selective Gram-negative media such as MacConkey agar. Because of the superficial resemblance of P. multocida to Haemophilus influenzae and Neisseria organisms, infections of the respiratory tract and central nervous system with this bacillus may be misdiagnosed initially.
Treatment of the patient with presumptive P. multocida infection should consist of careful washing of the wound and an attempt to leave it open. The empiric antibiotic of choice is amoxicillin/clavulanic acid orally for 7–10 days, with careful follow-up examination of the wound. P. multocida is susceptible to penicillin alone, and patients may be switched to penicillin if they have a pure infection with this organism. A fluoroquinolone, a tetracycline, and a trimethoprim-sulfamethoxazole are alternatives. In most patients, the antibiotic is aimed at both the patient’s skin flora (such as Staphylococcus aureus) and organisms from the animal’s mouth. Given the increasing prevalence of community acquired methicillin resistant S. aureus (MRSA), patients should be treated with vancomycin, clindamycin, tetracycline, or trimethoprim-sulfamethoxazole if MRSA is suspected (see Chapters 176 and 177).11
S. intermedius is an organism associated with dogs weighing more than 40 lb. It is more commonly found in canine gingival flora than is S. aureus (39% vs. 10%).12 It is a Gram-positive, coagulase-positive coccus that can be differentiated from S. aureus in the laboratory by biochemical testing. In comparison with S. aureus, S. intermedius does not produce acetoin from glucose and has β-galactosidase activity. If these tests are not performed (i.e., if only a latex assay is use to identify Staphylococci), the laboratory may incorrectly report an isolate as S. aureus or unspecified Staphylococcus. Antibiotic treatment is the same as that for infections with S. aureus, although resistance to oxacillin is uncommon in S. intermedius and is growing in importance for S. aureus.11
C. canimorsus is a capnophilic (“carbon dioxide-loving”), facultatively anaerobic, slow-growing, Gram-negative rod associated with dog bites.13–16 The organism has been found in the oral cavity of 17% of cats and 24% of dogs. Most infections occur in splenectomized or immunocompromised hosts, although fatal infections including meningitis have been reported in immunocompetent hosts and present as overwhelming sepsis.
Porphyromonas sp. are slow-growing anaerobic bacteria found in the deep gingival pockets of animal and humans. The organism is difficult to culture, requiring 4–6 days for colony formation as well as vitamin K and hemin in the media.17,18 Citron and co-workers19 found Porphyromonas sp. in 28% of culture specimens from patients with infected dog and cat bite wounds.
Bartonella henselae is the most common etiologic agent of cat scratch disease. Afipia felis and other Bartonella species may cause some cases (see Chapter 182).
(See Chapter 183).
(See Chapter 183).
(See Chapter 183).
The most notorious viral disease to occur after an animal bite is rabies, which is caused by a Rhabdovirus. Its epidemiology has changed in the past few years as a result of improved control of rabies in the domestic animal population. Now, nonimmune dogs and domestic animals account for only 8% of cases, whereas sylvatic animals, such as skunks, raccoons, red and gray foxes, and bats, represent the greatest potential danger. Rodents, such as squirrels and hamsters, are probably inconsequential as sources of rabies. However, cases of rabies in cattle are increasing.20 Postexposure prophylaxis, which is nearly 100% effective, has reduced the number of cases of human rabies from more than 100/year a century ago to an average of 1–4 cases a year in the United States today. In 2004, of the eight cases in the 49 states and Puerto Rico, four were acquired as a result of patients’ receiving transplanted organs from an infected donor.20,21 While in the United States death in humans from rabies is rare, WHO estimates that 55,000 deaths occur each year in developing countries, half in children under 15 years.22
Live virus is introduced into nerve tissue at the time of the bite, multiplies at the site, and then spreads to the central nervous system. It replicates in gray matter and then spreads along autonomic nerves to the salivary glands, adrenal glands, and heart. The incubation period varies with the site of the bite from 5 days to as long as several years. Clinical features include a prodromal period of 1–4 days, followed by high fever, headache, and malaise. Paresthesia at the site of inoculation occurs in 80% of patients. The next sequence of events is familiar: agitation, hyperesthesia, dysphagia, excessive thirst, paralysis, and death.
The diagnosis of clinical rabies is difficult and is often not made until after the death of the patient. Viral isolation methods may yield positive results for specimens of saliva or cerebral spinal fluid during the first 2 weeks of the illness, and saliva is infectious, so isolation precautions are needed. Serum antibodies may be detected as early as day 6 and usually by day 13. The fluorescent antibody method for detection of the viral antigen is the most rapid and sensitive means of making the diagnosis, and no matter where the actual bite site is, once patients have central nervous system disease, the diagnosis can be made from a biopsy of skin from the highly innervated hair-covered area of the neck or from brain tissue.
The most effective prevention for rabies is to avoid contact with any wild animal or any unfamiliar domestic animal. Persons at risk of unavoidable contact with rabies, such as spelunkers, veterinarians, virologists, and travelers spending time in countries where rabies is enzootic, should receive preexposure prophylaxis with the human diploid cell rabies vaccine (HDRV) series (three intramuscular injections on days 0, 7, and 21 or 28) given in the deltoid muscle and repeated every 2 years. In persons vaccinated intradermally (using a lower intradermal dose), the neutralizing antibody titer should be followed, because the immune response may not be fully protective.23
The need for postexposure prophylaxis can be determined by the answers to the following questions: (1) What is the status of animal rabies in the locale where the exposure took place? (2) Was the attack provoked or unprovoked? (3) Of what species and size was the animal? (4) What was the state of health and vaccination record of the animal? (5) Will the brain of the animal be examined within 48 hours? (6) Can the animal be effectively quarantined? Most animals transmit rabies virus in saliva only a few days before becoming ill themselves; bats, however, may harbor the virus for many months.
If the dog or cat responsible for the bite is healthy and available for observation for 10 days, the patient should not be treated unless the animal develops rabies. At the first sign of rabies in the animal, the patient should be treated with rabies immunoglobulin (RIG) and the vaccine HDRV. The symptomatic animal should be killed and tested as soon as possible. If the animal is rabid or suspected of being rabid, or if it does not have up-to-date vaccination records, the patient should be treated with RIG and HDRV (see Section “Specifics of Treatment”).24
All skunks, bats, groundhogs, foxes, coyotes, raccoons, bobcats, and other carnivores should be regarded as rabid unless laboratory test results prove negative. The patient should be treated with RIG and HDRV.
Bites by other animals (livestock, rodents, lagomorphs such as rabbits and hares, and ferrets) should be considered individually.23 Local and state public health officials should be consulted about the need for prophylaxis. Bites by squirrels, hamsters, guinea pigs, gerbils, chipmunks, rats, mice, and other small rodents almost never call for anti-rabies prophylaxis.
The most important step is to cleanse the wound immediately with a brush and soap to remove as much virus as possible. The wound should be rinsed well and then scrubbed a second time with green soap or 70% alcohol, which is rabicidal. If vaccine treatment is indicated, both RIG and HDRV should be given as soon as possible, regardless of the interval after exposure, unless the patient has been previously vaccinated and a serologic assay shows current immunity. In this case, only HDRV is needed and should be given on day 0 and day 3. For nonimmune hosts, administration of RIG is the most urgent. If HDRV is not immediately available, RIG should be started and HDRV given as soon as it is obtained. RIG (20 IU/kg) should be given immediately—50% around the site of the bite and 50% in the thigh or the arm. This passive immunization will result in the early appearance of antibody but will also inhibit development of the active antibody from HDRV, hence the need for a 28-day series. Active immunization is accomplished using HDRV, given intramuscularly for a total of five doses on days 0, 3, 7, 14, and 28. Serum for rabies antibody testing should be collected 2 weeks after the fifth dose. If there is no antibody response, an additional booster should be given.
In the rare cases in which a patient is admitted with the clinical diagnosis of rabies, several steps should be taken immediately. First, the diagnosis must be suspected and the patient placed on isolation precautions while the diagnosis is made rapidly by fluorescent antibody staining of specimens from various tissues, as well as immunofluorescence staining of the source animal’s brain tissue. Elevated antibody titers in the absence of immunization are clear evidence of infection. The first signs of clinical rabies are usually nonspecific, such as malaise, anorexia, fatigue, headache, and fever. The acute neurologic illness that follows is most commonly characterized by intermittent episodes of hyperactivity. In some cases, however, a progressive paralysis occurs. The usual period from onset of symptoms to onset of coma is 10 days. Risk of exposure for hospital staff includes contact with open wounds or mucous membranes with saliva or other potentially infectious material such as neurologic tissue, spinal fluid, or urine. Blood, serum, and stool are not considered infectious.
Basic clinical management consists of anticipating and preventing all treatable complications of the rabies infection and induction of coma to manage the patient until the patient generates an immune response.25 Pulmonary hypoxia should be prevented by tracheostomy at the first sign of respiratory difficulty, monitoring of actual partial pressure of oxygen, and use of supplemental oxygen. There are no specific antiviral treatments for rabies, although ribavirin has been tried.25 Anticonvulsant therapy should also be instituted. Extreme increases in intracranial pressure may be prevented by insertion of a cerebrospinal fluid reservoir that allows withdrawal of the intraventricular fluid and measurement of intracranial pressure. Cardiac arrhythmias may be anticipated with careful monitoring. Rabies had been regarded as uniformly fatal, but there have been patients who have survived with prolonged cardiorespiratory support, and there is serologic evidence that some animals have survived. An aggressive approach to treatment of the patient with known rabies infection is worthwhile. In 2004, a 15-year-old girl without a history of prior rabies vaccination survived after her physicians used a treatment based on induced coma as well as other supportive measures and antiviral agents.20,25 The World Health Organization has complied information regarding treatment and prevention accessible on their Web site.22
Herpesvirus simiae (B virus) is enzootic in Old World monkeys, especially rhesus, cynomolgus, and other macaques. The illness in monkeys is similar to that caused by herpes simplex in humans, and asymptomatic monkeys may shed the virus in saliva. Most cases in humans have occurred after direct exposure to macaque monkeys or monkey tissue through bites, scratches, or cuts.26 A vesicular lesion develops at the wound site with progressive lymphadenitis and fever. Over the next few days to weeks patients develop a severe illness, which is characterized by rapidly progressive ascending neuropathy and encephalitis. The illness is rare and before the availability of antiviral therapy, 72% of cases were fatal. A cluster of four cases occurring in 1987 in Pensacola, Florida, were treated with acyclovir. The two patients who received acyclovir when their infections were localized responded well to therapy and were maintained on oral acyclovir. All macaques should be presumed to be shedding B virus and should be handled accordingly. All monkey-inflicted injuries should be cleansed, specimens should be collected for viral culture, and a physician should evaluate the patient. Clinical and serologic monitoring is critical, and high-risk or infected patients should be treated according to established guidelines. The use of acyclovir or ganciclovir initially and acyclovir for years has led to improved survival in infected patients.
In the United States, there are approximately five to six deaths from snakebites and probably 6,000 to 7,000 snakebite envenomations each year.26 The largest number of venomous snakebites occur in the Southwestern States. Worldwide as many as 125,000 fatal snakebites may occur each year.27 Because of regional differences in varieties of snakes, management recommendations including specific antivenom recommendations differ by geographic area. The World Health Organization has published guidelines to help insure the quality and availability of antivenom immunoglobulins worldwide, and has an online database that can be used to explore the global distributions of venomous snake species, and access information on antivenom products and their manufacturers.28
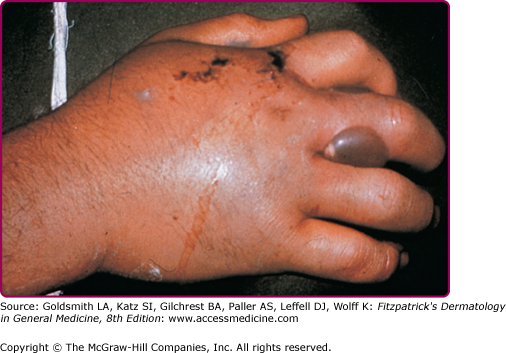
The two major poisonous snakes in the Americas are the pit viper (family Viperidae, subfamily Crotalinae, which includes the rattlesnake, water moccasin, and copperhead) and the coral snake (family Elapidae). Poisonous snakes are found in all states of the United States except Maine, Hawaii, and Alaska.29 The northern copperhead, also called the highland moccasin, is pink or reddish brown and is marked with large chestnut brown barrels resembling dumbbells or hourglasses (Fig. 209-1). The bite is painful but rarely fatal. The timber rattler is dark brown with chevrons of black and brown (Fig. 209-2).
The degree of toxicity of a snakebite depends on the potency of the venom, the amount injected, the size and condition of the snake, and the size of the person bitten. Pain occurs at the site of the bite (usually within 5 minutes). Signs and symptoms at the bite site include wheal with local edema, numbness, and, within moments, ecchymosis and painful lymphadenopathy (Fig. 209-3).
Nausea, vomiting, sweating, fever, drowsiness, and slurred speech may develop. Bleeding of the gums and hematemesis are common hemorrhagic manifestations. If edema and erythema have not developed within 8 hours after the bite, it can be assumed that significant envenomation did not occur. Estimated mortality rates for victims who receive antivenom in a health care facility are less than 1% and probably less than 0.1%.
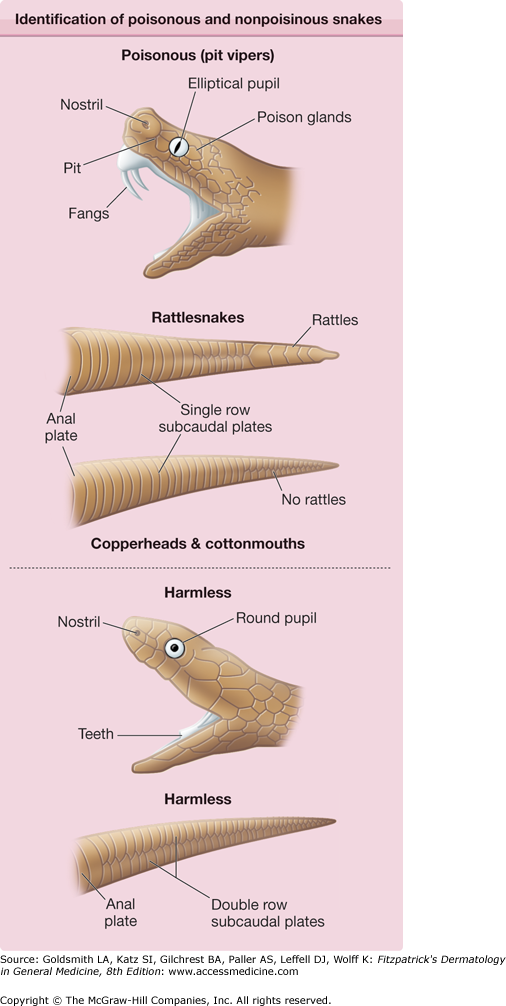
For proper treatment, it is extremely important to establish that the bite is from a poisonous snake. Many patients experience symptoms related to fear that may mimic symptoms from venom. The patient should have distinct fang punctures and immediate local pain, followed by edema and discoloration within 30 minutes. It is helpful to inspect the snake, because those that are poisonous may be differentiated from those that are not by the presence of fangs and the shape of the pupils (Fig. 209-4). Availability of a photograph of snakes common to a specific geographic area is important for all hospital emergency wards to aid practitioners in deciding if the patient has been bitten by a poisonous snake.
Although there are still insufficient evidence-based criteria for first aid and out-of-hospital treatment for snakebite envenomations, a rational approach is outlined in Table 209-1.30 In summary, the patient should be stabilized and transported as rapidly as possible to a health care facility capable of administering antivenom, and the affected site should be immobilized below the level of the heart. Constricting bands such as watches or jewelry should be removed; the routine use of venous compression bands is not recommended.29 The patient should be kept as immobilized as possible.29 Venous compression techniques are designed to impede the return of venous and lymphatic flow from the bite site without sacrificing blood flow to the extremity. In cases in which there is likely to be a significant delay in transport and the victim is developing systemic signs of envenomation such as hypotension, these techniques may be of value.30 Arguments against the use of venous compression techniques include the time that it takes to apply the treatments, which may further delay transport time. Additional arguments against the use of these techniques include the potential for concentrating the venom in the affected limb, which may worsen local necrosis, along with the possibility of a bolus effect when the compression is released.30 Another possible disadvantage of the use of these compression techniques is the potential for them to cause arterial compression if the affected limb swells in reaction to the toxin. If a decision is made to use a venous compression band in the field, arterial pulses should be palpable distal to the ligature, and the dressing should be checked frequently to make sure that it is has not become too tight. If a venous compression dressing has been applied in the field and is not causing vascular compromise, it should be left in place until the patient reaches a health care facility and antivenom is at the bedside.30
|
The role of surgical intervention in the treatment of snakebite envenomations is limited in most cases. Surgery is appropriate only if medical treatment fails. This includes elevation of the bitten body part in conjunction with the administration of four to six vials of Crotalidae polyvalent immune Fab (ovine) (FabAV) over the course of 1 hour, fails.29 Debridement of truly necrotic skin and subcutaneous tissues is recommended 4–5 days after envenomation. In cases of bites to a digit, if there is severe swelling and the finger is tense, blue, or pale, a digit dermotomy may be of use. True compartment syndrome involving an extremity is a rare complication of snakebite envenomation. Fasciotomy should be used only in cases of increased compartment pressure that has not responded to prompt and sufficient treatment with antivenom.31
The primary treatment for crotaline snake venom poisoning is prompt and adequate dosing with antivenom. Antivenom is most effective if given within 4 hours of the snakebite (Box 209-1).
|
FabAV should be used for all severe American crotaline snakebites, but not those of the coral snake. The dosage should be guided by the severity and progression of local changes and systemic clinical signs. Because some patients develop recurrent manifestations of envenomation after initial treatment, both a loading dose of four to six vials of FabAV and then, once initial control has been achieved, three maintenance doses 6, 12, and 18 hours later are recommended.29
Supportive treatment is indicated, including hospitalization with careful evaluation of baseline hematocrit, platelet count, and prothrombin time. Coagulopathy should be corrected by using antivenin, because fresh frozen plasma is ineffective.29 The wound should be cleansed and covered. Antitetanus therapy and antibiotic prophylaxis with penicillin, ampicillin/sulbactam, or tetracycline should be initiated for severe bites.
Bites and Stings of Aquatic Organisms
Normally, a seal bite occurs on the finger of a trainer or a seal hunter—thus the term seal finger or spaek finger.32,33 The etiologic agent is unclear and is controversial. One study suggests that a virus similar to that causing Orf may be the culprit,33 although Mycoplasma sp. have been isolated, and some patients respond to tetracycline (see Chapter 183). The incubation period of 4–8 days is followed by throbbing pain, erythema at the site, and swelling of the joint proximal to the bite. Untreated, seal finger progresses to cellulitis, tenosynovitis, and arthritis. Tetracycline, 500 mg orally four times a day for 10 days, remains the antibiotic of choice.30,32 It is also helpful to immobilize and elevate the finger as well as soak it several times a day (see Chapter 183).
Stings caused by jellyfish, Portuguese man-of-war, sea anemones, and corals are the most common envenomations experienced by humans in marine environments. All of these creatures are members of the phylum Cnidaria, formerly known as Coelenterata. They are divided into three major classes. The first class, Hydrozoa, includes the Portuguese man-of-war, fire corals, and hydroids. Jellyfish belong to the second class, Scyphozoa. The third class, Anthozoa, encompasses sea anemones and true corals. Approximately 100 of the 9,000 species of Cnidaria that have been identified may cause injury to humans.34
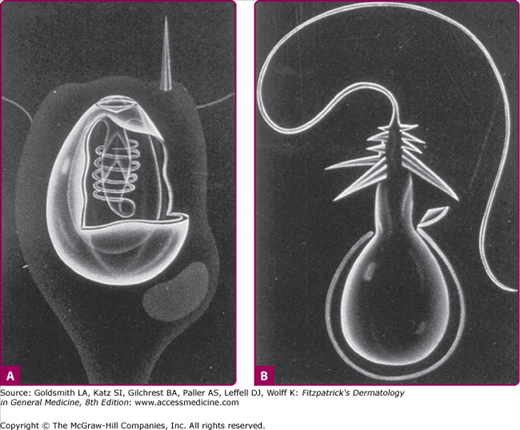
Cnidarians are radially symmetric animals with body walls formed by an inner and outer layer of cells enclosing a jelly-like substance. They may be either free floating in the water like jellyfish or sessile like corals.34 Almost all cnidarians possess nematocysts, or stinging capsules, which are usually concentrated on some form of tentacle. Each nematocyst contains a toxin or group of toxins and a coiled thread-like apparatus with a barbed end that functions like a flexible hypodermic syringe (Fig. 209-5). When the nematocyst comes into contact with a victim, the barbed end is discharged, and the toxin is injected into the skin.
Cnidarian stings range from mild, self-limited irritations to extremely painful and serious injuries, depending on the toxin of the species involved and the magnitude of the envenomation. Stings from certain species such as the cubomedusae, or box jellyfish, can be fatal.
In most cases, jellyfish stings elicit toxic reactions that may be localized and/or systemic. Although immediate-type hypersensitivity reactions, including urticaria, angioedema, and anaphylaxis, occur less frequently, they require prompt medical intervention, because shock and death may ensue in highly sensitized individuals. Allergic contact dermatitis, delayed and persistent hypersensitivity reactions, granuloma annulare, and erythema nodosum are other possible cutaneous reactions to jellyfish stings.35
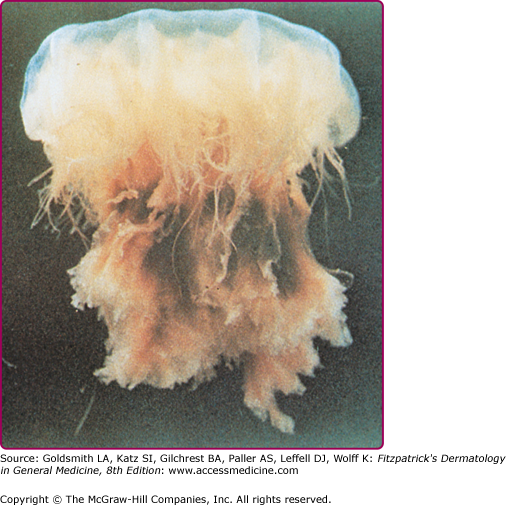
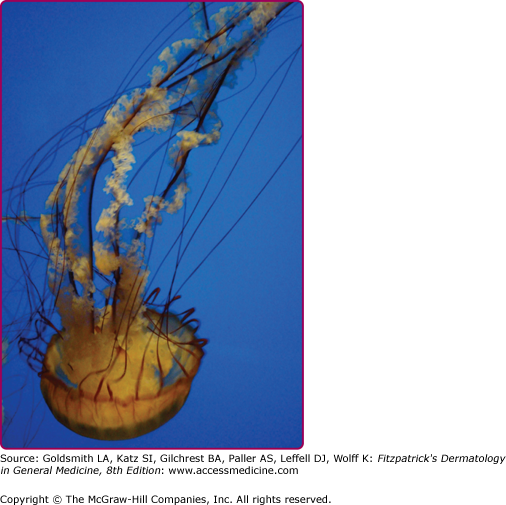
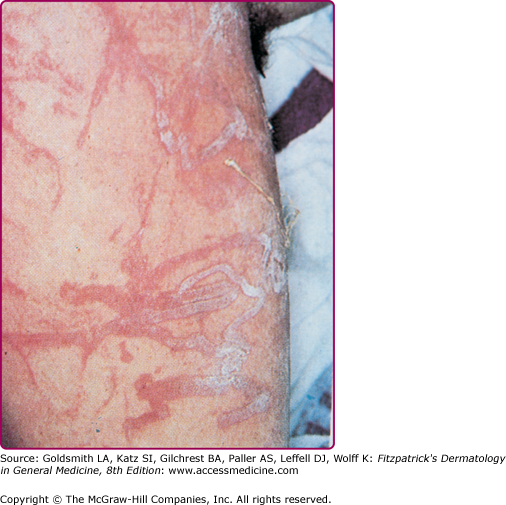
Among the organisms most commonly causing jellyfish stings are the sea nettles, which comprise two different species, both of which inhabit Atlantic as well as Indo-Pacific waters. Cyanea capillata and its relatives are the larger of the two species, with a bell measuring up to 1 m and numerous tentacles reaching 30 m in length (Fig. 209-6).36 Chrysaora fuscescens (Fig. 209-7) is found in the Pacific waters off the coast of California. Chrysaora quinquecirrha is smaller, with a white or rusty bell that may reach 30 cm with four digestive tentacles hanging from it. Although sea nettle stings are seldom lethal, they can be quite painful.37 Initially the victim experiences a sharp burning pain in the area contacted by the tentacles. Within minutes, the sting area develops a zigzag, whip-like pattern of raised red welts 2–3 mm wide (Fig. 209-8). The duration of acute pain may vary, but the pain often begins to abate in 30 minutes. The wheals usually subside by 1 hour, but purplish brown petechial and postinflammatory pigmentation may persist for several days.
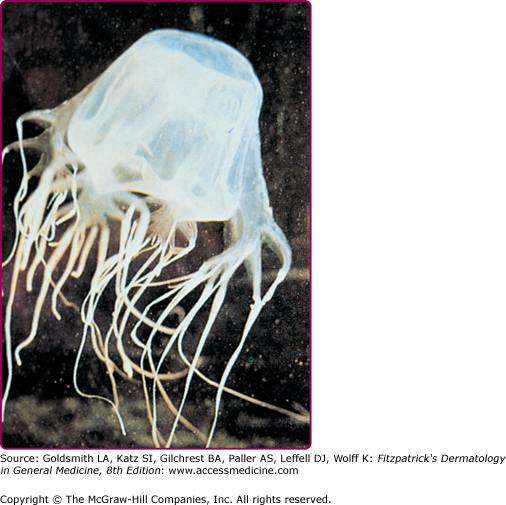
Physalia physalis is the species name for the Portuguese man-of-war, which is a member of the class Hydrozoa and is therefore not a true jellyfish. P. physalis is encountered in both Atlantic and Mediterranean waters, and is easily recognized by its translucent blue to pink or purple bladder-like float with multiple tentacles (Fig. 209-9). P. physalis is distinguished from its Pacific ocean relative Physalia utriculus, commonly known as the blue bottle, by its larger bell, which ranges in size from 10 to 30 cm, and multiple fishing tentacles extending up to 30 m; in contrast, P. utriculus has only a single tentacle that rarely exceeds 5 m.38 These tentacles are armed along their entire length with hundreds of thousands of nematocysts arranged in stinging batteries, with each battery containing hundreds of nematocysts. The nematocysts remain active even after portions of the tentacles are broken off in storms or when these animals are stranded on the shoreline by high winds or waves. A beached Portuguese man-of-war can cause a severe sting when stepped on or touched. Children who are stung after handling these animals and then cry and rub their eyes may develop an acute conjunctivitis.
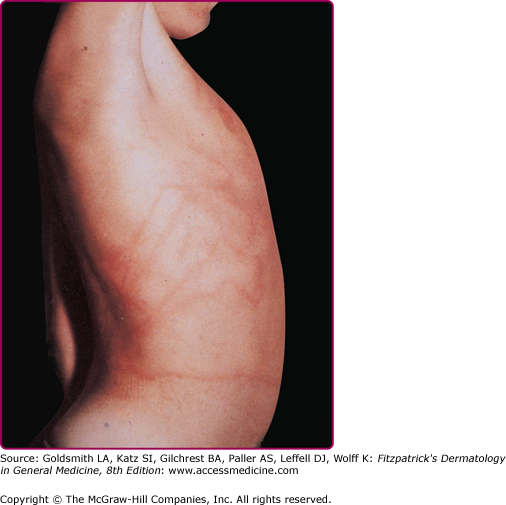
Stings of P. physalis are more painful and severe than those caused by sea nettles and are more extensive and serious than those caused by P. utriculus. At the moment of contact with the tentacles of P. physalis, the victim experiences a sharp, shock-like, burning pain. There may be painful paresthesias or numbness in the sting area. Initially, the sting area appears as an irregular single line or multiple lines composed of red papules, beaded streaks, or erythematous welts that correspond to the areas of tentacle contact. The wheals resolve in hours but may progress to vesicular, hemorrhagic, necrotic, or ulcerative stages before healing (Fig. 209-10).39
Postinflammatory striae may persist for weeks to months. Severe localized complications of P. physalis stings may also include arterial spasm in the sting site that can result in distal digital gangrene.40,41
Within 10–15 minutes of a Physalia sting, the victim may develop symptoms of an envenomation reaction characterized by nausea, abdominal cramps, muscular pains, backache, irritability, dyspnea, and chest tightness. Intravascular hemolysis and acute renal failure were reported in a 4-year-old girl after a severe sting by P. physalis.42 Most reports of death due to stings of P. physalis are not well-documented, but well-substantiated case reports of human fatalities do exist.43–45
Of all the species of jellyfish that cause painful stings and distress to swimmers, the species with the most established record of lethality are the cubozoans. Chironex fleckeri or box jellyfish causes at least one death each year in Australia.46 The fatality is usually a child, presumably because the size of the victim and the total area of the sting determine the likelihood of death. Until recently, most of the published cases of C. fleckeri stings involved fatal or nearly fatal envenomations, but less serious stings do occur in endemic areas.47–49
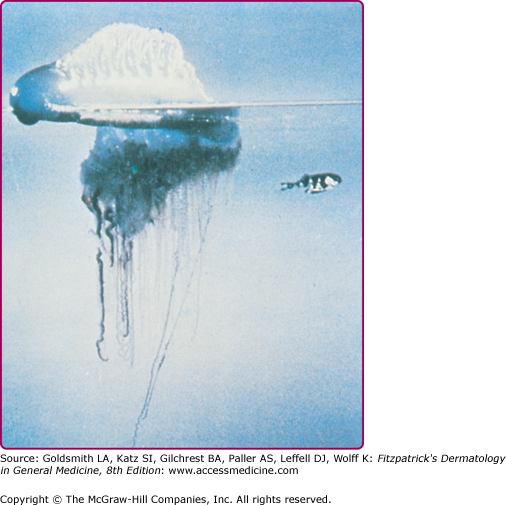
C. fleckeri (commonly known as the sea wasp) is an advanced species of jellyfish, with a semitransparent cubic bell that may grow to a volume of 9 L and weigh more than 6 kg (Fig. 209-11).46 Trailing from the bell are up to 60 stinging tentacles, which may reach 2–3 m in length. When a human comes into contact with a box jellyfish, some of the tentacles are torn off and adhere to the skin. Rescuers of C. fleckeri sting victims must exercise caution, because they also are at risk of envenomation until the tentacles have been neutralized and removed.
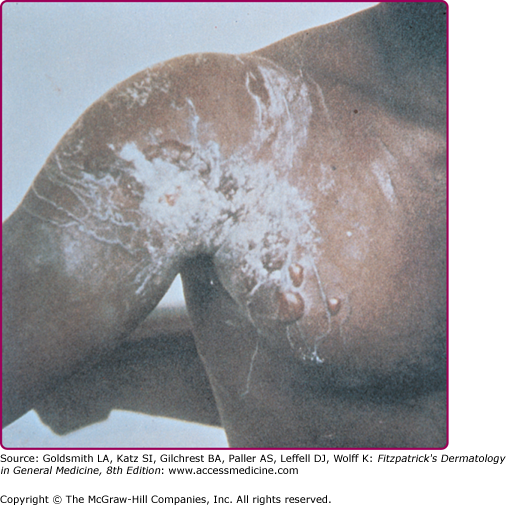
The stings appear initially as linear welts that give the patient the appearance of having been whipped.46 Fresh stings of C. fleckeri are easily recognized because they have a diagnostic, frosted, cross-hatched, or ladder-like appearance (Fig. 209-12). Microscopic diagnosis is also possible from blade scrapings or tape-strippings from the sting site. The intense pain may persist for many hours. Severely stung areas of skin take on a dusky cyanotic appearance and blister formation and necrosis may occur. The healing process is slow and may be complicated by bacterial superinfection and scarring.