Bilateral Sequential Lung Transplantation
Bassem N. Mora
David P. Mason
DEFINITION
Bilateral sequential lung transplantation involves the sequential removal of both recipient lungs with subsequent orthotopic implantation of donor lung allografts. This typically involves bilateral mobilization of the diseased lungs prior to arrival of the donor organs, followed by sequential recipient pneumonectomy and implantation of the donor lung allografts.
Donor lungs are most commonly procured following clinical brain death. In select cases, lung allografts can also be procured following donation after cardiac death (DCD). Living related lung transplantation has also been described, although its use in clinical practice is limited due to the need for two donors, a very small recipient, and potential for significant morbidity to the donors. It is rarely practiced in the United States due to recent changes to the lung allocation system.
PATIENT HISTORY AND PHYSICAL FINDINGS
In the United States, lung transplantation can only be performed at accredited transplant centers under the guidance of the United Network of Organ Sharing (UNOS). Posttransplant outcomes are reported annually to UNOS.
Each lung transplant candidate, following a thorough preoperative evaluation, is discussed in a multidisciplinary transplant meeting, reviewing indications for lung transplantation, contraindications, and alternative treatments.
Prior to the acceptance of donor lungs for transplantation, a thorough assessment of the brain-dead donor is carried out by the accepting transplant center. Multiple variables are reviewed, including the circumstances of brain death, potential contraindications to organ donation, cigarette use history, high-risk behavior history, age, ABO blood group compatibility, radiologic studies, and findings on bronchoscopy and the arterial blood gases.
Typical contraindications to organ donation include active malignancy, active cigarette use, advanced age, pulmonary infiltrates or pneumonia, pulmonary contusions compromising graft function, significant purulent secretions on direct bronchoscopic examination of the airways, and a PaO2 less than 300 mmHg on 100% FiO2.
IMAGING AND OTHER DIAGNOSTIC STUDIES
ABO compatibility is routine in lung transplantation. Tissue human leukocyte antigen (HLA) typing and cross-matching are considered with a “virtual” cross-match as well as with retrospective cross-matching.
For patients who are immunologically incompatible, plasma-pheresis with various desensitization protocols is an option.
A detailed pretransplant evaluation of the recipient is beyond the scope of this chapter. A number of laboratory tests are routinely ordered, including various hematology, coagulation, and chemistry blood tests; nicotine and cotinine levels; viral and fungal serologies; tumor markers; blood typing; and immune panels. Sputum is sent for routine culture and sensitivity for acid-fast bacilli and fungus.
Procedural studies include an electrocardiogram, echocardiogram, bone densitometry, bronchoscopy, left and right heart catheterization, esophagogastroduodenoscopy, colonoscopy, esophageal manometry, dobutamine stress testing, gastric emptying studies, and a barium swallow.
Pulmonary laboratory studies include pulmonary function testing, an arterial blood gas, spirometry, and a 6-minute walk test.
Radiologic studies include a two-view chest radiograph, quantitative lung ventilation-perfusion scan, high-resolution chest computed tomography (CT) scan with contrast, and a nasal sinus CT scan or abdomen and pelvis CT scan as indicated. Note the extensive reticular interstitial lung disease along with associated architectural distortion with fibrosis, traction bronchiectasis, and honeycombing.
A number of consults are arranged to determine fitness for transplantation including various medical specialties such as pulmonary medicine, thoracic surgery, gastroenterology, cardiology, otolaryngology, immunology, infectious disease, chemical dependency, psychiatry, and social work.
SURGICAL MANAGEMENT
Either single or bilateral lung transplantation can be performed depending on institutional preference and patient diagnosis. Some centers routinely perform bilateral lung transplantation in all patients due to the associated 25% increase in overall survival compared to unilateral lung transplantation seen in some patient populations.
Other centers, including ours, are more selective, given the scarcity of donor organs. Older patients with idiopathic pulmonary fibrosis and chronic obstructive lung disease are selectively offered single lung transplantation. Patients with pulmonary hypertension and infectious lung diseases typically undergo bilateral sequential lung transplantation.
Preoperative Planning
Routine preoperative laboratory studies and a chest radiograph are ordered on the day of the transplant procedure. Blood pressure monitoring is achieved by an arterial line placed prior to induction of anesthesia.
Operative informed consent should include a clear statement regarding the possibility of transmission of infectious disease or malignancy from the donor as well as whether the donor is considered a Centers for Disease Control and Prevention (CDC) high-risk donor.
Standard operative risks should be discussed, including but not limited to bleeding, infection, immunosuppression
complications, allograft dysfunction, airway or vascular anastomotic complications, need for prolonged mechanical ventilation and tracheostomy, acute and chronic rejection, need for mechanical circulatory support such as extracorporeal membrane oxygenation (ECMO), and death.
In patients who are certain to require cardiopulmonary bypass (CPB) during the operation, a single lumen endotracheal tube can be used. Patients undergoing lung transplantation without CPB are typically intubated with a left-sided, double lumen endotracheal tube to allow for selective lung ventilation during the procedure.
A Swan-Ganz pulmonary artery (PA) catheter with an introducer sheath for large-volume resuscitation is placed following induction of anesthesia, usually via the right internal jugular vein. The tip of the PA catheter is advanced just beyond the pulmonary valve, so as not to interfere with stapling of the PA at the time of recipient pneumonectomy. The presence of pulmonary hypertension may warrant CPB for the operation’s safe conduct. The perfusion team should be present in the operative suite throughout the procedure, as CPB may be needed emergently at any time.
A transesophageal echocardiogram is performed routinely in all patients to assess ventricular function, rule out associated cardiac anomalies, assist in de-airing at the end of the procedure, and assessing the vascular anastomoses.
A Foley urinary catheter is placed.
Perioperative antibiotics are center-specific. We typically employ cefuroxime for perioperative coverage in addition to vancomycin on a selective basis. Postoperative antibiotics are chosen based on donor and recipient airway cultures.
One gram of methylprednisolone is administered prior to reperfusion of the right lung allograft.
Positioning
Position the patient with both arms tucked. In the case of the clamshell incision, a vertical back roll is placed in order to further elevate the lateral chest off the operative table.
Mark the proposed incision on the patient. In the case of the clamshell approach, the 4th intercostal space is marked as well as the inframammary crease, particularly in women. In the case of a median sternotomy approach, the central part of the sternum is marked. Both femoral arteries are marked in case the patient requires postoperative ECMO support.
Widely prep and drape the chest, abdomen, pelvis, groins, and legs. The prep should extend laterally to the midaxillary line for the clamshell approach. An iodine-impregnated drape is placed over the skin.
A cell saver is used in all cases. This is helpful in blood salvage prior to and following CPB. A separate “dirty” suction catheter is used to suction the bronchus of airway secretions.
Especially in the case of inflammatory lung disease such as cystic fibrosis or bronchiectasis, frequent bronchoscopic suctioning of the airways by the anesthesia team is necessary.
TECHNIQUES
INCISION
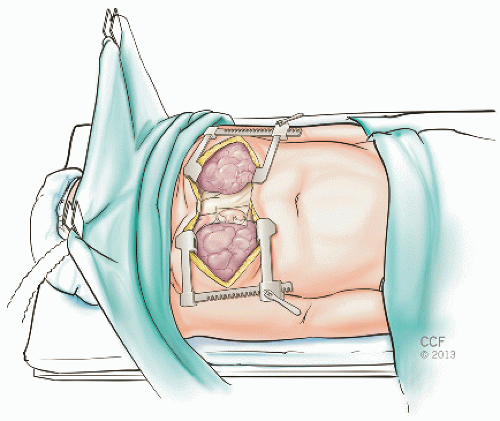
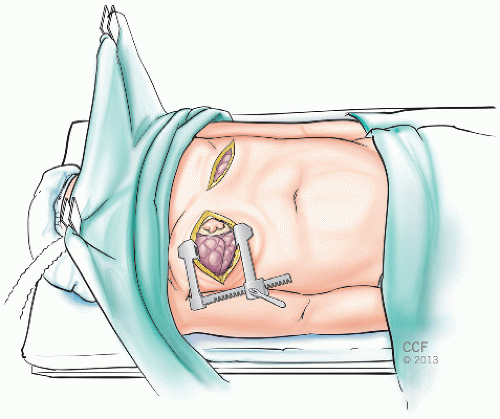
Our institutional preference is to perform bilateral sequential lung transplantation through either a trans-sternal bilateral anterolateral thoracotomy “clamshell” incision (FIG 1) or a median sternotomy, depending on surgeon preference. Alternatively, bilateral anterolateral thoracotomy incisions through the 4th intercostal space can be made, avoiding the need for sternal division (FIG 2). This limits surgical exposure but results in superior sternal stability. Two perpendicular retractors are used per side to expose the hilar structures. In the absence of sternal division, if CPB is needed, then either peripheral groin cannulation can be used or central cannulation of the right atrium and ascending aorta through the right thoracotomy.
In women, the anterolateral thoracotomy incision should be in the inframammary crease. Flaps should be elevated to allow entry into the 4th intercostal space. In large
breasted women, a retraction stitch is sometimes placed through the breast tissue and secured superiorly to the drape to allow better exposure.
In the setting of single lung transplantation, the 4th intercostal space is typically used. Due to anatomic differences between the right and left hilum, a posterolateral approach offers some advantage on the left side, whereas an anterior thoracotomy is almost always preferable on the right side.
In the case of a clamshell incision, following entry into the pleural space, the intercostal muscles are incised laterally with electrocautery to the paraspinal ligaments to allow less traumatic opening of the thoracotomy retractor. Both internal mammary artery pedicles are clipped, divided, and oversewn. Both pleural spaces are entered and bilateral thoracotomy retractors are placed.
Mediastinal structures should be mobilized prior to heparinization and CPB. Pericardial stay sutures are placed and the right and left pulmonary arteries and the right-sided pulmonary veins are mobilized.
CARDIOPULMONARY BYPASS
The routine use of CPB in bilateral lung transplantation is controversial. Most centers rarely use it for single lung transplantation unless there is intraoperative hemodynamic instability. In the setting of bilateral lung transplantation, some centers use CPB selectively and others routinely.
We prefer to use CPB for the majority of bilateral lung transplants. We believe this results in more stable hemodynamics, faster recipient pneumonectomy and implantation, quicker blood salvage through cardiotomy suckers, and avoidance of exposure of the ischemic first lung allograft to the entire cardiac output while the second lung allograft is implanted. Disadvantages include the potential for systemic inflammatory response, the need for arterial and venous cannulation, and the potential for increased postoperative bleeding from acquired coagulopathy and increased transfusion requirement.
The use of a median sternotomy incision, in contradistinction to a clamshell incision, requires CPB in all cases because exposure of the left hilum is otherwise impossible without decompression of the heart. Likewise, if concomitant cardiac surgical procedures are to be performed, then CPB is necessary.
The institution of CPB should be done when the procurement team is en route to the operative suite. After full heparinization, arterial cannulation is accomplished through the ascending aorta. Venous cannulation is via a two-stage cannula in the right atrial appendage or via bicaval cannulae in the superior and inferior vena cava, depending on surgeon preference.
Right Hilar Mobilization
A suture can be placed on the diaphragm to retract it caudally and further expose the right hilar structures. Release the inferior pulmonary ligament up to the inferior pulmonary vein. Care should be taken to avoid bleeding at the most caudal aspect of the ligament where a small arterial blood vessel is invariably present.
Use scissors to mobilize the hilum, with occasional electrocautery. Clips are applied generously on small bronchial and lymphatic vessels to avoid postoperative bleeding and lymphatic leak. Mobilization of the recipient lungs is more difficult in the setting of prior surgery or adhesions, and appropriate coordination with the donor team is of paramount importance to avoid unnecessary delays in allograft implantation.
The right inferior pulmonary vein (RIPV) is mobilized and so is the right superior pulmonary vein (RSPV) and right main pulmonary artery (RMPA). The RMPA is superior to the RSPV as well as anterior to the right mainstem bronchus.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree