30 Benign and malignant nonmelanocytic tumors of the skin and soft tissue
Synopsis
 All typical skin and skin-associated soft-tissue tumors, apart from malignant melanocytic tumors (malignant melanoma), are described from the point of view of a plastic surgeon.
All typical skin and skin-associated soft-tissue tumors, apart from malignant melanocytic tumors (malignant melanoma), are described from the point of view of a plastic surgeon.
 Biopsies should only be performed for a clear purpose, such as for the differential diagnosis of benign tumors or to analyze the stage and grade of malignant tumors, which would allow the area to be resected to be determined.
Biopsies should only be performed for a clear purpose, such as for the differential diagnosis of benign tumors or to analyze the stage and grade of malignant tumors, which would allow the area to be resected to be determined.
 One current and useful model is the reconstructive matrix, which helps plastic surgeons to determine the best reconstructive solutions for their patients in the context of particular medical and socioeconomic environments by considering aspects of surgical complexity, technological sophistication, and patient surgical risk.
One current and useful model is the reconstructive matrix, which helps plastic surgeons to determine the best reconstructive solutions for their patients in the context of particular medical and socioeconomic environments by considering aspects of surgical complexity, technological sophistication, and patient surgical risk.
Introduction
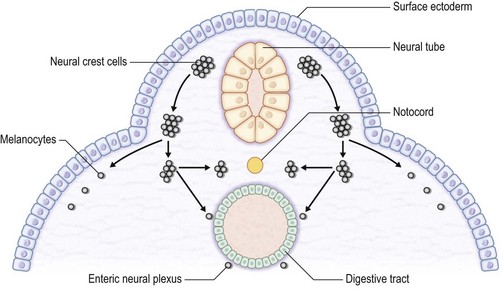
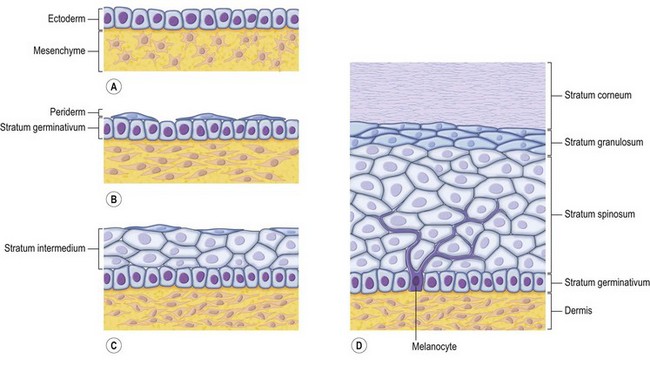
The skin consists of the epidermis, which is derived during ontogeny from the superficial ectoderm, and the dermis, which is derived from the mesenchyme. Starting in the first 3–4 weeks of human ontogeny, cells derived from the neural crest migrate into the epidermis (Fig. 30.1) where they become melanocytes and Schwann cells; the latter associate with peripheral nerves in the skin. Later, the cutaneous appendages develop. These include hair, which originates from epidermal cells, and hair papillae, which are filled with mesenchyme; vessels and peripheral nerve endings also develop in the papillae. Other cutaneous appendages are the sebaceous glands, which are derived from the epithelial wall of the hair papillae, and the eccrine and apocrine sweat glands, which are also epidermal in origin. There are also soft tissues that are associated with skin, namely fat, muscles, and blood vessels (all of which have a mesenchymal lineage) and nerves (derived from neural crest cells). Thus, skin and skin-associated soft-tissue tumors can be classified simply into those that are of epithelial, cutaneous appendage, neural crest, and mesenchymal origin (Fig. 30.2).
Diagnosis
Inspection and palpation
The diagnosis of skin tumors starts with inspection and palpation. The following information should be recorded: the number of lesions (solitary or multiple), the shape of the lesions (e.g., round, oval, polygonal, geographic, linear, annular), its size, its elevation status (e.g., narrow-pedicled, wide-pedicled, dome-like, hemispherical, flat elevated, umbilicated), its surface status (e.g., smooth, rough, papillary, granular, transudatory, xerophily, ulcerative, erosive, atrophic, lustrous, necrotic), its color (e.g., normal, yellow, pale yellow, erythematous, blackish brown, black, blue, depigmented, pigmented, hyperemic, livid), its hardness (e.g., soft, elastic soft, elastic hard, hard, bone-like hard, fluctuating), its alignment (e.g., localized, disseminated, centrifugal, systematized, singular, symmetric, asymmetric, bilateral), its site, whether there are any subjective symptoms (e.g., pain, itch, contracture sensation, numbness, burning sensation, cold sensation), and the time course of the appearance of the lesion (e.g., acute, subacute, chronic, temporary, recurrent). Color plays a particularly important role in the diagnosis of skin lesions (Box 30.1). The possibility of malignant tumors should be suspected at all times. If the shape, size, elevation status, or color of a lesion changes rapidly, a biopsy should be considered.
Dermoscopy
Dermoscopy is a specialized technique that employs a binocular microscope to observe the skin surface. It is useful for diagnosing pigmented lesions and is necessary for differentially diagnosing malignant melanoma and nevus. It is also required for the diagnosis of seborrheic keratosis, basal cell carcinoma (BCC) and vascular lesions. The Consensus Net Meeting on Dermoscopy 20001 has recommended the use of a two-step diagnostic algorithm, in which the first step is to differentiate melanocytic from nonmelanocytic pigmented lesions by using specific dermoscopic criteria. The second step is to differentiate between the various nonmelanocytic lesions by using other specific dermoscopic criteria (Fig. 30.3). The dermoscopic criteria that are used to diagnose melanocytic lesions, seborrheic keratosis, BCC, and vascular lesions are shown in Box 30.2.
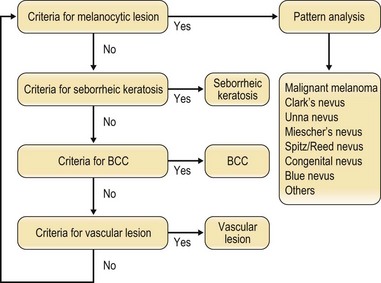
Fig. 30.3 Two-step diagnosis using dermoscopy. BCC, basal cell carcinoma.
(Modified from Consensus Net meeting on Dermoscopy. Available at http://www.dermoscopy.org/consensus/.)
Box 30.2
Dermascopic criteria used to diagnose melanocytic lesions, seborrheic keratosis, basal cell carcinoma, and vascular lesions
(Reproduced from the Consensus Net meeting on Dermoscopy. 2000 Available at http://www.dermoscopy.org/consensus/.)
Ultrasound and Doppler imaging
To observe skin lesions, high-frequency ultrasound around 20–50 MHz is needed.2 However, if the lesion shows extension perpendicular to the skin surface that exceeds a depth of 20 mm, the standard ultrasound (3–10 MHz) should be used. In such cases, computed tomography (CT) and magnetic resonance imaging (MRI) can provide additional information. Ultrasound can be used to determine tumor thickness, its relationship with adjacent structures, and the presence of lymph node metastasis. A variety of ultrasound devices are suitable for this purpose, including mechanical or electron scanning, one-dimensional A-mode, two-dimensional B-mode, and three-dimensional C-mode devices.
X-ray, CT, MRI, angiography, scintigraphy, and positron emission tomography (PET)
PET is also useful for detecting the metastatic lesions of malignant skin tumors.3 2-deoxy-2-[18F] fluoro-d-glucose (FDG) PET (FDG-PET) has been used for the diagnosis and staging of cancers and for monitoring treatment, particularly with regard to Hodgkin’s lymphoma, non-Hodgkin lymphoma, and lung cancer. PET can also sometimes detect many other types of solid tumors that occasionally show up as very highly labeled lesions. FDG-PET is particularly useful for searching for tumor metastasis, or for recurrence after the removal of a primary tumor that was known to be highly active. However, since PET can also detect inflammatory lesions, it will be necessary to exclude the possibility that a PET-detected apparent tumor is not an inflammatory, erosive, or ulcered area.
Pathologic diagnosis
A way to detect lymph node metastasis that is increasingly being used is to perform sentinel node biopsy, which shows whether the cancer has spread to the very first lymph node.4 If the sentinel lymph node does not contain cancer, it is highly likely that the cancer has not spread to any other area of the body. However, this technique is only of therapeutic value for patients with positive nodes: for patients who have negative nodes, the possibility that the lymph node has undetectable cancerous cells should be considered. In addition, there is no compelling evidence that the survival of patients who have a full lymph node dissection as a result of a positive sentinel lymph node biopsy is better than that of those patients who do not have a full dissection until later in the disease, when the lymph nodes can be felt by a physician. Thus, such patients may be subjected unnecessarily to full dissection, which is associated with lymphedema.
The TNM clinical classification system and the pTNM pathologic classification system
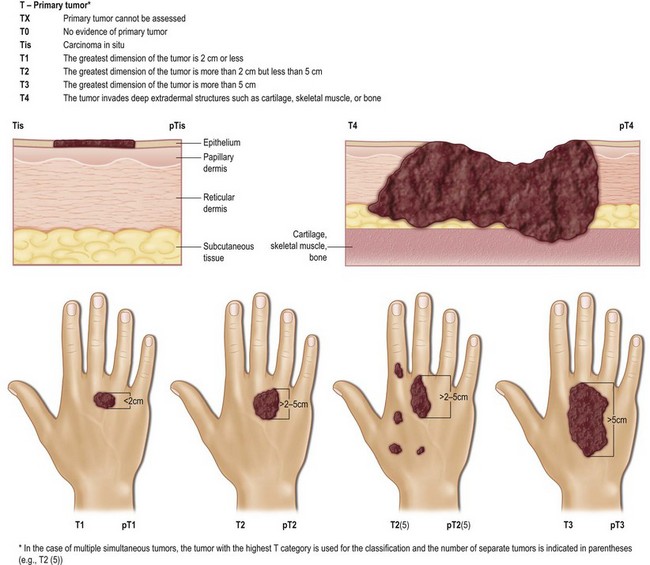
The TNM clinical classification5,6 applies only to malignant tumors (Figs 30.4, 30.5). T indicates the size of the tumor and whether it has invaded nearby tissue, N indicates whether regional lymph nodes are involved, and M indicates the presence of distant metastasis. The TNM classification system is based on clinical evidence acquired before definitive treatment. Once intraoperative and surgical pathologic data become available, the pathologic TMN (pTNM) classification system can be used. The pT, pN, and pM categories correspond to the T, N, and M categories.
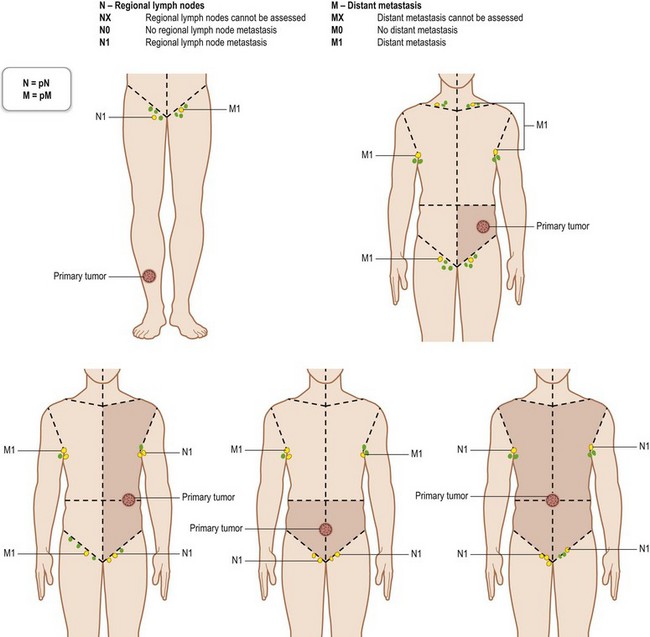
Regional lymph nodes are those that drain the site of the primary tumor (Fig. 30.6). The pN assessment of the regional lymph nodes requires that a sufficient number of lymph nodes are removed for histologic examination (usually six or more). If the examined lymph nodes are negative but fewer than six lymph nodes were resected, the pN classification is designated pN0.
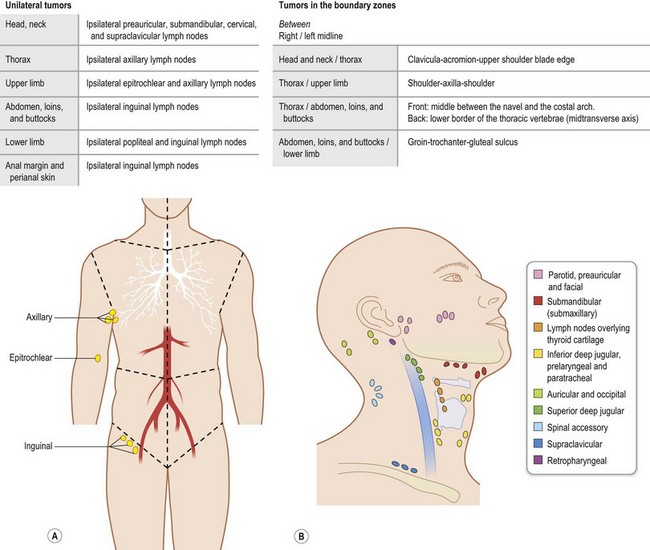
Fig. 30.6 The regional lymph nodes, as described by the International Union for Cancer (UICC).
(Used with the permission of the Union for International Cancer Control (UICC), Geneva, Switzerland. The original source for this material is TNM Atlas: Illustrated Guide to the TNM/pTNM Classification of Malignant Tumours 5th edition. Edited by Christian F. Wittekind, Frederick L. Greene, Robert V.P. Hutter, Martin Klimpfinger, Leslie H. Sobin. Springer, 2004, ISBN: 9783540442349.)
Box 30.3 shows examples of the TNM classification system, namely the systems used for eyelid, vulva, penis, and soft-tissue sarcomas.
Box 30.3
The TNM classification systems for eyelid, vulva, penis, and soft-tissue sarcomas (International Union for Cancer)
Carcinoma of the skin of the eyelid
TX Primary tumor cannot be assessed
T0 No evidence of primary tumor
Carcinoma of the skin of the vulva
TX Primary tumor cannot be assessed
T0 No evidence of primary tumor
T3 The tumor invades the lower urethra, vagina, and/or anus
NX Regional lymph nodes cannot be assessed
N0 No regional lymph node metastasis
N1 Unilateral regional lymph node metastasis
Carcinoma of the skin of the penis
TX Primary tumor cannot be assessed
T0 No evidence of primary tumor
Ta Noninvasive verrucous carcinoma
T1 The tumor invades the subepithelial connective tissue
T2 The tumor invades the corpus spongiosum or cavernosum
T3 The tumor invades the urethra or prostate
T4 The tumor invades other adjacent structures
NX Regional lymph nodes cannot be assessed
N0 No regional lymph node metastasis
N1 Metastasis in a single superficial inguinal lymph node
N2 Metastasis in multiple or bilateral superficial inguinal lymph nodes
N3 Metastasis in deep inguinal or pelvic lymph nodes, unilateral or bilateral
Soft-tissue sarcoma
TX Primary tumor cannot be assessed
T0 No evidence of primary tumor
T1 The greatest dimension of the tumor is 5 cm or less
T2 The greatest dimension of the tumor is more than 5 cm
(Modified from Sobin LH, Gospodarowicz MK, Wittekind C (eds) TNM Classification of Malignant Tumours (UICC: International Union Against Cancer), 7th edn. New Jersey: Wiley-Blackwell, 2009; and Wittekind CF, Greene FL, Hutter RVP et al. (eds) TNM Atlas: Illustrated Guide to the TNM/pTNM Classification of Malignant Tumours, 5th edn. Heidelberg: Springer, 2004.)
* Superficial tumors are located exclusively above the superficial fascia and do not invade the fascia, while deep tumors are either exclusively beneath the superficial fascia, or are superficial to the fascia but invade into or through the fascia. Retroperitoneal, mediastinal, and pelvic sarcomas are classified as deep tumors.
Clinical staging
The TNM system5,6 is used to show the anatomical extent of malignant tumors. For purposes of tabulation and analysis, it is useful to condense these categories into stages (Table 30.1). To be consistent with the TNM system, carcinoma in situ is categorized as stage 0. In general, tumors that are localized to the organ of origin are categorized as stages I and II, while those that exhibit extensive local spread, particularly to the regional lymph nodes, are classified as stage III. The tumors that have distant metastasis are classified as stage IV. For pathological stage groups, if sufficient tissue has been removed for pathological examination to evaluate the highest T and N categories, M1 may be either clinical (cM1) or pathological (pM1). However, if only a distant metastasis has had microscopic confirmation, the classification is pathological (pM1) and the stage is pathological.
Treatment
Wide excision
With regard to wide resection of malignant tumors of skin, the horizontal and vertical margins vary depending on the type of tumor. Retrospective histopathologic studies have supported reductions in the horizontal margin in recent years. The horizontal margins that are recommended for particular tumor types are listed in (Box 30.4). In the case of malignant soft-tissue tumors, the type of excision can vary with regard to the extent of the margins around the tumor: curative wide, wide, and marginal excisions indicate margins that are at least 5 cm outside the tumor-reactive layer, less than 5 cm outside the tumor-reactive layer, and within the tumor-reactive layer, respectively. Moreover, Mohs micrographic surgery, where tumors undergo histologic analysis during surgery in such a way that almost all of the surgical margins can be examined for tumor extensions, can help to obtain complete margin control during the removal of a skin cancer and soft-tissue sarcoma.7
Box 30.4
Recommended surgical margins of wide excisions of nonmelanocytic tumors of the skin and soft tissues
Reconstructive surgery
Reconstruction via skin grafting is a basic surgical technique that is used to reconstruct the tissue defects that occur after tumor extirpation, that is also useful for early detection of local recurrence. The most ideal approach after tumor extirpation is to suture the wound margins directly together. However, this can only be performed if the wound is not too big and the adjacent skin can be extended sufficiently. One concern with this approach is that malignant cells may be left in the deep margins of the wound. Recent developments in reconstructive techniques, including thin flap-based techniques and wound coverage materials, mean that plastic surgeons can now choose from a wide and rapidly evolving variety of primary and aesthetic secondary reconstruction methods. Given this constantly altering medical (and social) environment, it is difficult to develop up-to-date reconstructive algorithms, and indeed, previous algorithms such as the reconstructive ladder, elevator, and triangle quickly lose favor. Consequently, the techniques that are chosen for primary and secondary reconstruction are largely determined on a case-by-case basis. However, one current and useful model is the reconstructive matrix,8 which helps plastic surgeons to determine the best reconstructive solutions for their patients in the context of particular medical and socioeconomic environments by considering aspects of surgical complexity, technological sophistication, and patient surgical risk.
Radiation therapy
Malignant tumors vary in their sensitivity to radiation-induced damage, which directly affects the success of radiation therapy. For example, malignant melanomas are less sensitive to radiation and are therefore rarely treated with radiation therapy. Malignant skin tumors that are relatively sensitive to radiation and are therefore commonly treated with radiation therapy include BCC,9 SCC,10 and Merkel cell carcinoma of the skin.11 Acute skin reactions to radiation therapy occur during the first 7–10 days after treatment and are characterized initially by erythema that then progresses to pigmentation, epilation, and desquamation; this is particularly the case when higher doses are used. Subacute and late complications occur several weeks after radiation therapy and can progress for long periods of time. These complications include scarring, permanent pigmentation, depigmentation, atrophy, telangiectasis, subcutaneous fibrosis, and necrosis.
Chemotherapy
Chemotherapy can be either adjuvant or primary, and all chemotherapies can be administered either systemically or topically. Adjuvant chemotherapy is mainly used for malignant melanoma, while primary chemotherapy is indicated for SCC,12 angiosarcoma,13 and EMPD.14 However, the radical chemotherapy with which malignant skin tumors are generally treated can sometimes be seen as neoadjuvant chemotherapy before surgery for advanced-stage cancer. Single-agent chemotherapies include peplomycin sulfate and CPT-1115 for SCC, pacitaxel for angiosarcoma, and docetaxel for angiosarcoma and EMPD. Multiagent chemotherapies includes cisplatin + doxorubicin and cisplatin + 5-fluorouracil (5-FU) + bleomycin for SCC; mesna + doxorubicin + ifosfamide + dacarbazine for angiosarcoma; and 5-FU + mitomycin C, 5-FU + carboplatin + leucovorin, and 5-FU + carboplatin + mitomycin C + epirubicin + vincristine for EMPD.
Laser therapy
Dye lasers or neodymium-doped yttrium aluminum garnet (Nd: YAG) lasers can be used for lesions that are characterized by neoplastic changes or malformations in capillary vessels or their overgrowth, such as hemangiomas,16 vascular malformations,17 and keloid/hypertrophic scars.18 Ruby and alexandrite lasers can be used to treat superficial pigmented lesions whose colors range from brown to black, while Q-switched ruby and alexandrite lasers are useful for intradermal pigmented lesions such as the nevus of Ota.19 CO2 lasers and erbium yttrium aluminum garnet (Er: YAG) lasers target the water contents of a lesion,20 which makes them suitable for lesions with various colors like seborrheic keratosis, telangiectatic granuloma, xanthoma, and fibroma.
Benign cutaneous and soft-tissue tumors
Benign epithelial-origin tumors
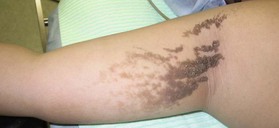
Epidermal nevus (e.g., verrucous epidermal nevus and linear epidermal nevus)
This nevus is composed of skin cells that normally occur at the affected site but show hyperkeratosis and papillomatosis (Fig. 30.7). It can be considered to be a hamartoma, which is a benign focal, tumor-like malformation that is composed of a mixture of the cells that characterize the tissue of its origin; such nodules grow at the same rate as the surrounding tissues. The dermis below the epithelial nevus is usually normal. Epithelial nevi sometimes exhibit a diffuse or extensive distribution that affects a large area of the patient’s body (termed systematized epidermal nevus); careful observation is necessary in these cases. Systematized epidermal nevus often occurs together with abnormalities in other organ systems. This condition is termed epidermal nevus syndrome,21 and has been described as a sporadic neurocutaneous linkage of congenital ectodermal defects in the skin, brain, eyes, and/or skeleton. Laser therapy, cryotherapy, electrocoagulation, surgical abrasion, and excision are suitable for treating epithelial nevi. If abrasion therapy is used, it should be remembered that these lesions are usually limited to the epidermis; thus, to prevent heavy scarring, only the epidermis and the superficial layer of the dermis should be removed.
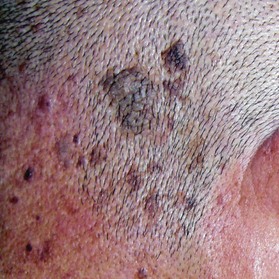
Seborrheic keratosis (also known as senile wart)
This is a benign skin growth that originates from the basal and squamous cells in the epidermis (Fig. 30.8). It should be differentiated from nevus cell nevus, senile keratosis, BCC, and malignant melanoma. The sign of Leser–Trélat, which is the dramatic, sudden appearance of multiple seborrheic keratoses, can be a paraneoplastic syndrome, namely an ominous sign of an internal malignancy.22 In such cases, not only do new lesions suddenly appear, pre-existing lesions also frequently increase in size and become symptomatic. This sign should not be overlooked and screening for internal malignancy should be recommended to the patient. Laser therapy, cryotherapy, electrocoagulation, surgical abrasion, and excision are all suitable treatments for seborrheic keratosis. However, if the tumor invades the dermis, which can occur, surgical excision is recommended.
Keratoacanthoma
Keratoacanthoma has been a controversial entity for many years, mainly because it closely resembles SCC23 (Fig. 30.9). It grows rapidly and can sometimes self-heal. Upon histopathology, atypical squamous cells can be detected, which makes it difficult to distinguish from SCC. For this reason, excisional biopsy should be considered despite the fact that this lesion occasionally self-heals. If the lesion is on the nose and face, Mohs micrographic surgery is particularly suitable since it facilitates good margin control along with minimal tissue removal.
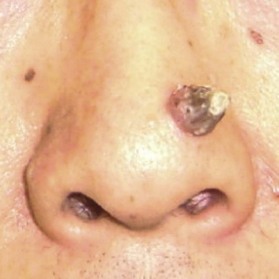
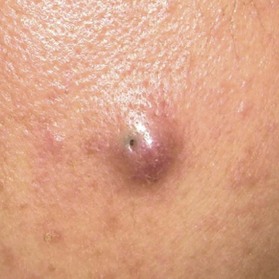
Epidermoid cyst (also known as epidermal cyst and atheroma)
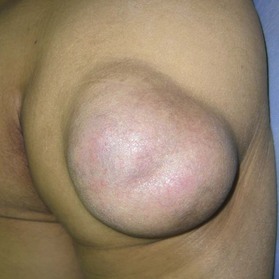
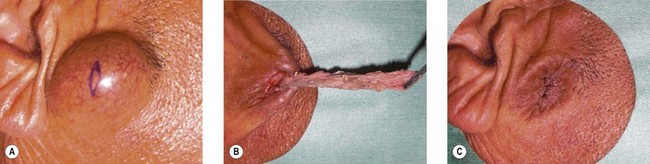
Epidermal cyst is a smooth, dome-shaped, freely movable, somewhat fluctuant subcutaneous swelling that is sometimes attached to the skin by a central pore (Fig. 30.10). It is covered with a stratified squamous epithelium that resembles the epidermis or the follicular infundibulum; thus, there is a granular cell layer adjacent to the keratin-containing cyst lumen. Epidermoid cysts can rupture spontaneously or be ruptured by external mechanical forces. Extremely large epidermoid cysts, also known as giant atheromas (Fig. 30.11), should undergo pathology to rule out malignant change,24 although such changes are rare. Epidermoid cysts that exhibit inflammation or recur should be removed by simple excision. In the case of large cysts, the contents can be removed first, after which the cyst walls can be removed with minimal incision (Fig. 30.12). In cases where pus and blood are excreted, the surgeon should consider incising the cyst and draining it first, and then excising it completely 1–2 weeks later.
Milia
Milia are a smaller version of an epidermoid cyst (less than 4 mm in diameter). They may derive from the outer root sheath of vellus follicles. There are primary and secondary milia.25 Primary milia include congenital milia, benign primary milia of children and adults, milia en plaque, nodular grouped milia, multiple eruptive milia, nevus depigmentosus with milia, and genodermatosis-associated milia. Secondary milia are the disease-, medication-, and trauma-associated milia. Milia can be treated easily by making small holes in the surface with a needle or CO2 laser and then extruding the contents.
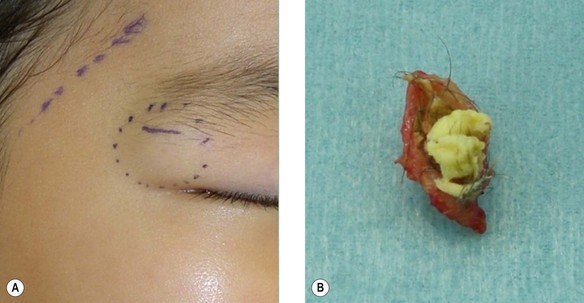
Dermoid cyst
A dermoid cyst is a congenital subcutaneous cyst that develops along the embryonic lines of closure (Fig. 30.13). It is most common on the head and neck area, particularly the supraorbital region, brow, upper eyelid, glabella, and scalp. These cysts can be easily removed surgically, but care should be taken not to injure the temporal branch of the facial nerve. The cyst lumen contains keratin debris and hair shaft fragments. Preoperative X-rays should be taken to distinguish it from pilomatricoma on the head and neck region, especially in pediatric patients. Since it has been reported that dermoid cysts can exhibit malignant changes, complete surgical removal is recommended.26