Bacterial Diseases
Greg K. Sakamoto
Tracie Chong
Jacob P. Thyssen
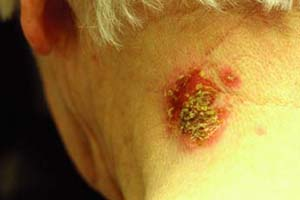 |
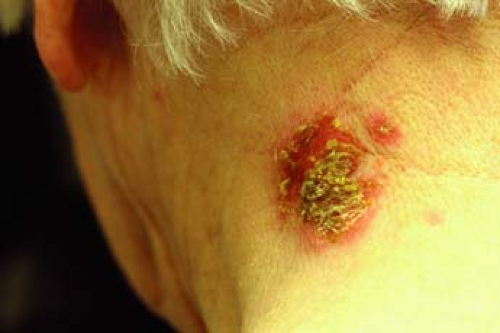
A 64-year-old woman presents to the clinic with a 2-week history of crusted erosions on the posterior neck. Upon examination, there is a 3-cm crusted plaque with honey-colored crusts (Fig. 5-1). She otherwise feels well with no local or systemic symptoms. She has no blisters on her skin or mucous membranes. What is the most likely diagnosis? What diagnostic tests should be done? What should the treatment be?
Exposure to bacteria is ubiquitous in the human experience, with millions of organisms being present in and on the body. In many cases, the relationship between bacteria and the human being is commensal but in some important cases, infection is pathogenic. These bacterial skin infections are important to recognize, diagnose correctly, and treat promptly. In this chapter, common skin infections will be discussed.
Key Features
There are two forms of impetigo: bullous and nonbullous.
Impetigo is primarily caused by infection by Staphylococcus aureus and group A β-hemolytic streptococci.
The most commonly affected group is children. Adults at risk for impetigo are those with diabetes mellitus, acquired immunodeficiency syndrome (AIDS), and the homeless. Bullous impetigo tends to occur more commonly in neonates.
Treat with topical mupirocin initially (at least 7 days), in widespread cases or when clinically necessary, add appropriate systemic therapy.
Impetigo
Background/Epidemiology
Impetigo is a highly contagious superficial skin infection. It is the most common bacterial skin infection in children between the ages of 2 and 5. Although mainly affecting children, adults are also susceptible to infection, especially those with diabetes mellitus, AIDS, and the otherwise immunosuppressed. The incidence tends to increase during the summer months. Infections are typically prevalent among nurseries, day care centers, and elementary schools.
Patients are able to autoinoculate themselves, leading to spread of the infection to other areas of the body. The organism is transmitted through direct skin contact and enters through any breach in the skin barrier. Once it invades, it causes an infection of the superficial epidermis that can manifest as two different subtypes: nonbullous and bullous impetigo. Bullous impetigo tends to occur more commonly in neonates.
Pathogenesis
Impetigo is among one of several cutaneous infections caused by Staphylococcus aureus and to a lesser extent, group A β-hemolytic streptococci. The nonbullous type accounts for 70% of the cases and is an immune-mediated condition. The bullous form is toxin-mediated and the cleavage of the epidermis is mediated by exfoliative toxins A and B, which disrupt cellular adhesion. Exfoliative toxin A and B are known to target desmoglein I, which is a desmosomal cadherin that is a key structure in intercellular adhesion in the epidermis. The result is a split in the granular layer of the epidermis with the formation of a thin-roofed blister.
Clinical Presentation
Nonbullous impetigo usually begins as a macule or papule that quickly becomes vesicular or pustular. The vesicle is easily ruptured and leads to the characteristic “honey-colored” or “golden” crust overlying the erosions of the skin (Fig. 5-1). Skin surfaces such as the perioral region and nares are frequently affected. Autoinoculation can lead to a linear or grouped arrangement of these vesicles. Most patients have skin lesions only, without systemic symptoms. In a minority of cases, regional lymphadenopathy may occur. Impetigo is usually a self-limited process that resolves in about 2 weeks without scarring or postinfectious sequelae. Five to ten percent of the cases are caused by S. pyogenes and can result in an acute poststreptococcal glomerulonephritis. Use of systemic antibiotics does not prevent nephritis.
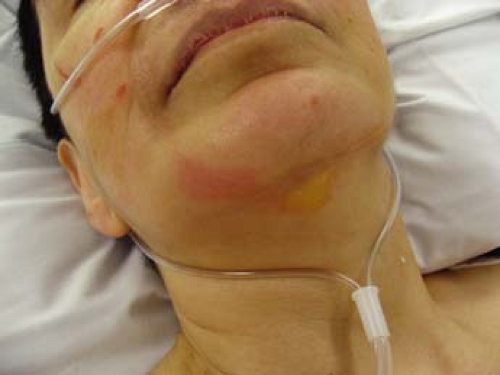
Bullous impetigo typically begins as large, flaccid bullae on the trunk or extremity with no surrounding erythema as seen in Figure 5-2. It favors the intertriginous regions such as the axillae, crural folds, and neck folds. When the bullae are ruptured, they ooze yellow serous fluid that forms the characteristic yellow, “honeycombed” crust. Because the bullae are fragile, erosions are often seen in place of ruptured bullae. The erosions usually have a collarette of scale at the periphery signifying previous bullae that have been unroofed. Similar to nonbullous impetigo, most cases are self-limited and resolve in 1 to 2 weeks without scarring. Systemic symptoms when seen include fever, weakness, and diarrhea. In rare cases, bullous impetigo has been associated with meningitis, pneumonia, sepsis, and even death.
Diagnosis
Most cases are diagnosed on the basis of clinical presentation. When the diagnosis is in question, a Gram stain and culture of the blister fluid may reveal the pathogenic bacteria. Sensitivities to antibiotics may also be required to identify methicillin-resistant S. aureus (MRSA) strains and determine appropriate antibiotic coverage.
Differential Diagnosis
Nonbullous impetigo:
Contact dermatitis
Herpes simplex
Varicella
Dermatophytosis
Bullous impetigo:
Bullous erythema multiforme
Bullous pemphigoid
Pemphigus vulgaris
Stevens-Johnson syndrome
Therapy
Although impetigo is thought to be a self-limited condition, treatment is usually instituted to prevent the progression of lesions and systemic involvement
as well as prevent the spread to others. If the area of involvement is limited, topical antibiotics remain the mainstay of treatment. Mupirocin is the most effective topical antibiotic and should be applied twice daily for at least 7 days. It was found to be effective in clearing MRSA strains and is routinely used in decolonizing patients who harbor MRSA. Systemic antimicrobials are the treatment of choice for widespread infections and infections with systemic symptoms. Beta-lactamase resistant antibiotics such as cephalexin, dicloxacillin, and amoxicillin/clavulanate should be used. Azithromycin and clarithromycin are also efficacious, but more costly. If the strains are methicillin resistant, then vancomycin, linezolid, and quinupristin/dalfopristin should be used. Other antibiotics that are effective against MRSA include trimethoprim-sulfamethoxazole, minocycline, and clindamycin. In case of MRSA, supplementary treatments should be initiated (e.g., hot washing of all towels, bed sheets, and clothes the patient has used).
as well as prevent the spread to others. If the area of involvement is limited, topical antibiotics remain the mainstay of treatment. Mupirocin is the most effective topical antibiotic and should be applied twice daily for at least 7 days. It was found to be effective in clearing MRSA strains and is routinely used in decolonizing patients who harbor MRSA. Systemic antimicrobials are the treatment of choice for widespread infections and infections with systemic symptoms. Beta-lactamase resistant antibiotics such as cephalexin, dicloxacillin, and amoxicillin/clavulanate should be used. Azithromycin and clarithromycin are also efficacious, but more costly. If the strains are methicillin resistant, then vancomycin, linezolid, and quinupristin/dalfopristin should be used. Other antibiotics that are effective against MRSA include trimethoprim-sulfamethoxazole, minocycline, and clindamycin. In case of MRSA, supplementary treatments should be initiated (e.g., hot washing of all towels, bed sheets, and clothes the patient has used).
When the diagnosis is not clear or the possibility of another more serious infection or blistering disorder is being entertained, the patient should be referred to a dermatologist for evaluation. This may include a bacterial Gram stain and culture, direct immunofluorescence antibody testing, viral culture, or skin biopsy. In severe cases with bullous lesions, frozen section biopsies may be indicated.
In widespread/severe cases where Stevens-Johnson syndrome (SJS), toxic epidermal necrolysis (TEN) or staphylococcal scalded skin syndrome (SSSS) is a possibility, emergent inpatient evaluation and care is indicated. These conditions can be life threatening and progress rapidly. See Chapter 23 for further discussion of SJS/TEN.
“At a Glance” Treatment
First-line therapy for isolated lesions: mupirocin ointment or cream twice daily for at least 7 days
First-line therapy for widespread infections and infections with systemic symptoms: beta-lactamase resistant antibiotics such as cephalexin, dicloxacillin, and amoxicillin/clavulanate azithromycin or clarithromycin
MRSA outpatient: based on sensitivities, empirically—minocycline, clindamycin, or trimethoprim-sulfamethoxazole
MRSA inpatient: vancomycin, linezolid, and quinupristin/dalfopristin
Course and Complications
Impetigo is usually acute and self-limited to 1 to 2 weeks. The lesions usually resolve without any complications or evidence of scarring. However, neonates have a higher incidence of developing a generalized infection as well as pneumonia and meningitis. If no improvement is seen while on antibiotic therapy, antibiotic sensitivities should be obtained to rule out drug-resistant strains. Other potential complications include endocarditis, cellulitis, guttate psoriasis, toxic shock syndromes, and staphylococcal scalded skin syndrome.
MRSA infection should always be ruled out by bacterial culture and sensitivities.
In widespread cases, consider SSSS or SJS/TEN.
ICD9 Codes
| 684 | Impetigo |
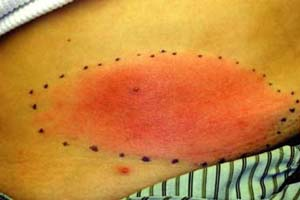 |
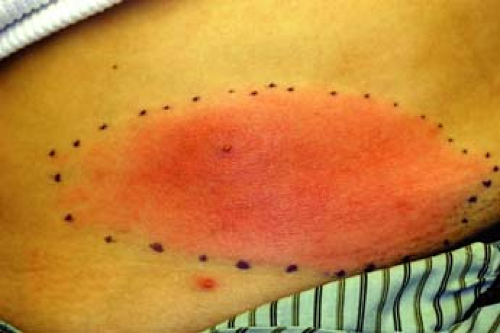
A 28-year-old woman presents with a lower abdomen lesion with ill-defined, nonpalpable borders; erythema (Fig. 5-3); warmth; pain; and swelling. Regional lymph nodes are palpable. She is not diabetic or immunocompromised. History includes recent shaving of the pubic hair prior to a 2-day history of fever, chills, and malaise. We tell her that clinical diagnosis points toward a skin infection. What organism is the most likely cause in this case?
Cellulitis
Background/Epidemiology
Cellulitis, a painful spreading infection in the deep dermis or subcutaneous tissue, is one of the most common bacterial skin infections. By definition, it does not involve muscle or deeper structures. It can occur anywhere on the body, but most commonly affects the lower limbs and digits, followed by the face, feet, hands, torso, neck, and buttocks. It frequently occurs in the settings of minor trauma, alcoholism, diabetes mellitus, intravenous drug use, peripheral vascular disease, and damage to the lymphatic
system. Those with interdigital tinea pedis, onychomycosis, or impetigo are more susceptible to chronic lower extremity cellulitis.
system. Those with interdigital tinea pedis, onychomycosis, or impetigo are more susceptible to chronic lower extremity cellulitis.
Key Features
Common bacterial infection of the dermis, most often due to Streptococcus pyogenes (group A β-hemolytic streptococci) or Staphylococcus aureus in immunocompetent adults.
Lower limbs and digits most commonly affected.
Treat with oral antibiotics with gram-positive coverage.
Pathogenesis
Cellulitis is most often due to Streptococcus pyogenes (group A β-hemolytic streptococci) or Staphylococcus aureus in immunocompetent adults. Most cellulitis in childhood is associated with S. aureus, because infection by Haemophilus influenza has become less common since vaccine implementation. Cellulitis in diabetic patients (generally surrounding diabetic ulcers) is polymicrobial, caused by gram-positive cocci and gram-negative aerobes and anaerobes. Damage to the skin barrier is often the mechanism of infection in immunocompetent patients, while a hematogenous route is likely the source in the immunocompromised.
Clinical Presentation
Cellulitis generally follows a period of systemic symptoms such as fever, chills, and malaise. The area exhibits induration and typical signs of inflammation (erythema, warmth, pain, and swelling). Upon examination, the lesion has ill-defined, advancing, nonpalpable borders such as that seen in Figure 5-3. Severe cases may present with vesicles, bullae, pustules, or necrotic tissue. Infection may progress to include ascending lymphangitis and regional lymph node involvement. Location of cellulitis varies by age and demographics: children usually show involvement of the head and neck, while the extremities are often affected in adults. Cellulitis in intravenous drug abusers commonly presents in the upper extremities.
Diagnosis
Diagnosis of cellulitis is generally based on clinical presentation. Leukocyte count is typically normal or slightly elevated, and blood cultures are nearly always negative (except in immunocompromised individuals). The exception is infection with H. influenza, which presents with an elevated leukocyte count with left shift and positive blood cultures. Atypical organisms are common in children and the immunocompromised. Such cases may necessitate needle aspiration and skin biopsy for routine pathology and tissue culture.
Histopathologic analysis is usually not required but will typically show inflammatory infiltrate of lymphocytes and neutrophils throughout the dermis, which may extend into the subcutaneous fat. There may also be edema and dilation of lymphatics and small blood vessels, and subepidermal bullae may be noted in cases of severe dermal edema. Special stains may elucidate the causative organism, but organisms are rarely found in cellulitic lesions because immunocompetent cells rapidly eliminate most viable bacteria by the time of clinical presentation. Involvement simultaneously of both lower extremities (“bilateral cellulitis”) is exceedingly rare and other diagnoses such as stasis dermatitis or allergic contact dermatitis must be considered.
Therapy
Treatment includes immobilization, elevation, wet dressings, and analgesics. Antibiotic treatment is aimed against S. pyogenes and S. aureus. The
majority of cases may be given a 10-day course of an oral antibiotic with gram-positive coverage. More serious cases or patients with facial cellulitis may require hospitalization and parenteral antibiotics. Parenteral antibiotic therapy should include penicillin along with a penicillinase-resistant penicillin such as cloxacillin to cover S. aureus. Broad-spectrum coverage such as an aminoglycoside plus clindamycin or an advanced-generation cephalosporin is needed in diabetic or decubitus ulcers complicated by cellulitis.
majority of cases may be given a 10-day course of an oral antibiotic with gram-positive coverage. More serious cases or patients with facial cellulitis may require hospitalization and parenteral antibiotics. Parenteral antibiotic therapy should include penicillin along with a penicillinase-resistant penicillin such as cloxacillin to cover S. aureus. Broad-spectrum coverage such as an aminoglycoside plus clindamycin or an advanced-generation cephalosporin is needed in diabetic or decubitus ulcers complicated by cellulitis.
If there is no improvement following 24 to 36 hours of treatment, cultures and sensitivities should be ordered and antibiotics may be adjusted appropriately. Although anti-inflammatory agents may enhance resolution of the infection, NSAIDs are generally avoided in the treatment of cellulitis as they may mask the signs and symptoms of deeper necrotizing infections.
“At a Glance” Treatment
First-line: recommended basic care such as immobilization, elevation, wet dressings, and analgesics
For uncomplicated cellulitis, consider a course of oral antibiotic with gram-positive coverage such as cephalexin or dicloxacillin
Facial cellulitis or cases with rapidly spreading disease/constitutional illness may require inpatient care
Course and Complications
Cases of superficial cellulitis generally improve within several days, but some patients with dermal thickening may take several days of parenteral antibiotics for significant improvement to occur. Although complications are infrequent, acute glomerulonephritis, lymphadenitis, and bacterial endocarditis may result. Damage to lymphatic vessels may lead to lymphedema and increased risk of recurrent infection.
Consider referral when the diagnosis is uncertain or if the cellulitis fails to respond to antibiotics within the expected time period.
Severe blistering should be emergently evaluated.
Deep vein thrombosis.
Stasis dermatitis.
Superficial thrombophlebitis.
Panniculitis.
Allergic or irritant contact dermatitis.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree