Axillary Sentinel Lymph Node Biopsy
Julie A. Margenthaler
Historical Considerations
The method and role of axillary staging for breast cancer has been in evolution since the 1960s. Historically, the prognostic information derived from axillary lymph node dissection was viewed so important to the overall patient management that clinically and pathologically uninvolved lymph nodes were removed for staging and treatment planning, though the therapeutic benefit was questionable. There has been a long-standing interest in the pathways of metastasis, with special emphasis placed on the draining regional lymphatics. The sentinel node concept was an elegant hypothesis founded on the principle that the lymphatic vessels draining a specific primary tumor travel to the first, or a finite group of several, “sentinel,” lymph nodes in the respective regional lymphatic basin before disseminating to the remaining nonsentinel lymph nodes (1). Researchers at the John Wayne Cancer Institute (2,3) first demonstrated the sentinel node hypothesis in an animal model and subsequently validated it in a group of patients with melanoma. Following the success of intraoperative lymphatic mapping for melanoma, the technique of sentinel lymph node biopsy was quickly adapted to early-stage breast cancer (1).
Indications
The presence of metastatic disease in the axillary lymph nodes is considered the single most important prognostic factor for patients with breast cancer, whereby patients have a poorer prognosis with increasing numbers of metastatic lymph nodes (4). Sentinel lymph node biopsy has emerged as the standard method of axillary staging in breast cancer patients with clinically negative axillas (1,5,6,7). The goal of sentinel lymph node biopsy is to minimize morbidity while maintaining high sensitivity and a low false-negative rate, such that axillary staging is similar to the standard provided by axillary lymph node dissection pathology. Early experience with the procedure revealed a wide variation in reported rates of successful mapping and accuracy. However, training and quality control programs were initiated which have resulted in a large number of surgeons capable of performing sentinel lymph node biopsy in a standardized fashion with
a high degree of pathologic accuracy (7). The National Surgical Adjuvant Breast and Bowel Project (NSABP) B-32 trial was primarily designed to demonstrate whether sentinel lymph node biopsy resulted in a mortality difference or differences in regional control compared to those patients undergoing axillary lymph node dissection. No significant differences were observed. In addition, the NSABP B-32 data were secondarily evaluated to demonstrate whether sentinel lymph node biopsy could achieve the same diagnostic accuracy as the gold standard, axillary lymph node dissection, while significantly decreasing morbidity (7). In more than 5,600 patients enrolled in NSABP B-32, the sentinel lymph node(s) were identified successfully in 97.2% of patients, with an overall accuracy of 97.1% (95% confidence interval [CI], 96.5 to 97.8) and a false-negative rate of 9.7% (95% CI, 7.6 to 11.9). The negative predictive value was 96.1% (95% CI, 95.2 to 97.0) (7).
a high degree of pathologic accuracy (7). The National Surgical Adjuvant Breast and Bowel Project (NSABP) B-32 trial was primarily designed to demonstrate whether sentinel lymph node biopsy resulted in a mortality difference or differences in regional control compared to those patients undergoing axillary lymph node dissection. No significant differences were observed. In addition, the NSABP B-32 data were secondarily evaluated to demonstrate whether sentinel lymph node biopsy could achieve the same diagnostic accuracy as the gold standard, axillary lymph node dissection, while significantly decreasing morbidity (7). In more than 5,600 patients enrolled in NSABP B-32, the sentinel lymph node(s) were identified successfully in 97.2% of patients, with an overall accuracy of 97.1% (95% confidence interval [CI], 96.5 to 97.8) and a false-negative rate of 9.7% (95% CI, 7.6 to 11.9). The negative predictive value was 96.1% (95% CI, 95.2 to 97.0) (7).
Contraindications
Absolute and relative contraindications for the use of sentinel lymph node biopsy have been proposed, including prior mastectomy, prior axillary surgery or previous sentinel lymph node biopsy, palpable lymphadenopathy, prior excisional breast biopsy, T3 and T4 tumors, male breast cancer, neoadjuvant chemotherapy, and multicentric/multifocal breast cancers. However, as surgeon experience with sentinel lymph node biopsy has progressed, the previously described contraindications for sentinel lymph node biopsy have been successfully challenged. T4 and inflammatory breast cancer remain contraindications.
Imaging
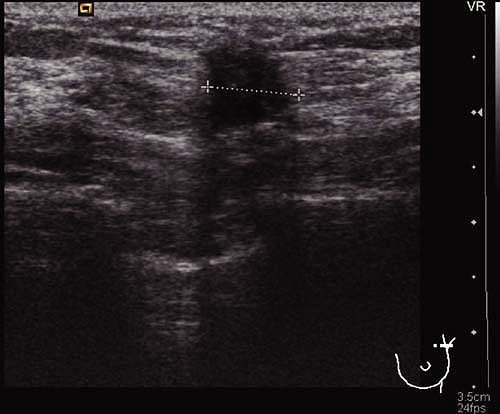
Prior to definitive surgical axillary staging, axillary ultrasound may be used to visualize the axillary lymph nodes. At our institution, this has become the standard for all newly diagnosed breast cancer patients with clinically negative axillae. Multiple reports in the literature suggest that axillary ultrasound is a potentially valuable technique for identifying axillary metastases (8). Axillary ultrasound permits the visualization of lymph node size, shape, contour, and changes in cortical morphology and texture that appear to be associated with the presence of axillary metastases (Figs. 10.1 and 10.2). However, sonographic signs of metastatic disease sometimes overlap with those of benign reactive changes, limiting the ability of this modality alone to accurately stage the axilla. The addition of fine needle aspiration biopsy has been shown to increase the specificity of nodal staging (9). If the patient is confirmed to have axillary nodal metastases by
cytopathology, full axillary lymph node dissection is recommended. However, if the axillary lymph nodes are morphologically normal or if the cytopathology is negative or nondiagnostic, sentinel lymph node biopsy should be performed.
cytopathology, full axillary lymph node dissection is recommended. However, if the axillary lymph nodes are morphologically normal or if the cytopathology is negative or nondiagnostic, sentinel lymph node biopsy should be performed.
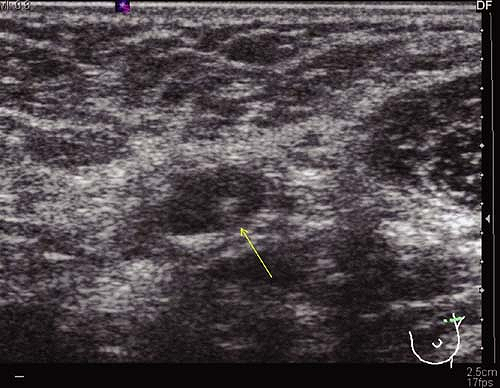 Figure 10.1 Axillary ultrasound of a normal-appearing lymph node. The node has a smooth, homogenous cortex with a centrally located, preserved fatty hilum (arrow). |
Lymphoscintigraphy
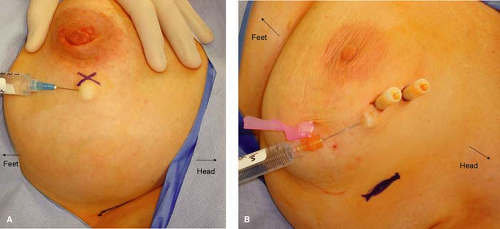
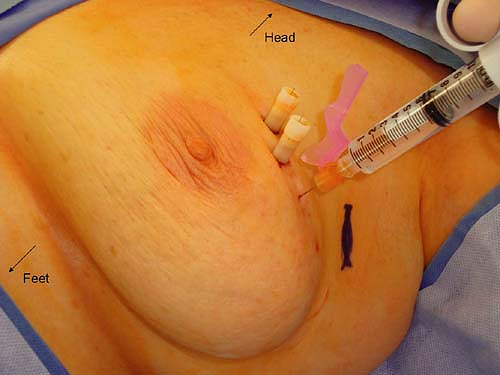
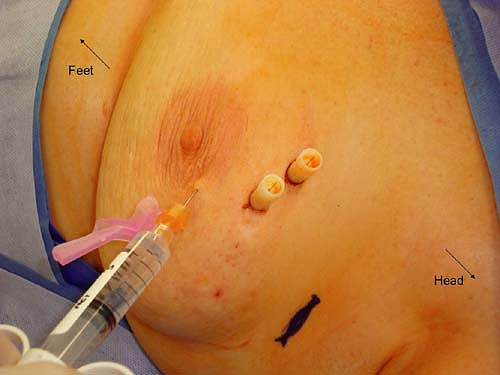
Mapping and identification of the sentinel lymph node can be accomplished using radioactive colloid injection with or without lymphoscintigraphy and/or vital blue dye injection. For most surgeons who utilize radioactive colloid injection, the injection is performed by the nuclear medicine specialists at the institution prior to the planned surgical procedure. The recommended guidelines include an injection of approximately 0.5 mCi of 0.2-μm-filtered technetium sulfur colloid. This injection has traditionally been performed approximately 1 to 2 hours prior to the sentinel lymph node biopsy procedure. However, the method of injection varies widely. Options for site of injection include peritumoral versus subareolar and options for method of injection include intraparenchymal versus intradermal. A recent prospective randomized trial was performed to compare these modalities. Patients were randomized to receive radiocolloid injection by an intradermal route (placed in the skin overlying palpable tumors or in the same quadrant near the nipple areolar border for nonpalpable tumors), an intraparenchymal route (administered in a peritumoral fashion), or a subareolar route (at the upper, outer edge of the areolar complex directed medially 5 mm below the complex). Figures 10.3 to 10.5 depict the described injection methods. Intraoperative identification rates were 90% or more for all methods (100% for intradermal, 95% for subareolar, and 90% for intraparenchymal), further supporting the notion that sentinel lymph node biopsy is a robust technique regardless of the site and/or method of injection (10). However, the mean time to first localization on lymphoscintigram was 8 ± 14 minutes for intradermal injection, 53 ± 49 minutes for intraparenchymal injection, and 22 ± 29 minutes for subareolar injection (10). On the basis of these results, we have adopted the intradermal method of injection at our institution. Anecdotally, this has been very successful, and we now require our patients to present to the nuclear medicine department approximately 45 minutes prior to their surgical procedure, rather than 2 hours which was our previous practice with intraparenchymal injections.
Recently, Thompson et al. (11) reported their experience with intraoperative subareolar injection of radioactive sulfur colloid by the operating surgeon. They found that this was a safe, effective, and equally reliable method of identification of the sentinel lymph nodes. Further, intraoperative injection of the colloid avoided the patient pain, vasovagal events, operative delays, and costs associated with preoperative injections. One potential disadvantage of the intraoperative method of injection is the lack of access to lymphoscintigraphy. This may be important if the surgeon typically relies on the
lymphoscintigram for information regarding number of localized nodes, position of localized nodes in the axillary space, and presence of internal mammary sentinel lymph nodes.
lymphoscintigram for information regarding number of localized nodes, position of localized nodes in the axillary space, and presence of internal mammary sentinel lymph nodes.
Vital Blue Dye
The injection of a vital blue dye can be used alone or in conjunction with radioactive colloid injection for sentinel lymph node mapping. The vital blue dye is universally injected intraoperatively and will be discussed further in the technique section. However, options also exist for the type and method of blue dye injection planned. The issues of the site of blue dye injection are similar to those for radioactive colloid injection. Peritumoral or subareolar injections may be employed, though many surgeons have adopted subareolar and periareolar injections because of their simplicity and convenience. Further results of previous studies have validated the efficacy of subareolar and periareolar injections, supporting the hypothesis that the lymphatic drainage of the entire breast is to the same few sentinel lymph nodes (12).
The two available vital blue dyes for injection and sentinel lymph node localization are isosulfan blue dye and methylene blue dye. Isosulfan blue is a triphenylmethane-based dye and is one of the rosaniline dyes. It was the first blue dye of its class to be approved for use in lymphangiography by the Food and Drug Administration under the trade name Lymphazurin 1% (US Surgical Corp, Norwalk, Connecticut). Typically, 5 mL of Lymphazurin 1% is used for injection. Although isosulfan blue dye remains a popular choice of dye for sentinel lymph node localization, the rate of allergic reactions to the dye has also increased (13




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree