Arterial Reconstruction in Children
Jaimie D. Nathan
Brian S. Pan
Greg M. Tiao
DEFINITION
Hepatic arterial reconstruction in pediatric orthotopic liver transplantation is defined as the creation of vascular anastomoses for arterial inflow to the liver allograft. Arterial reconstruction in children is technically challenging, as pediatric recipients have a higher risk of hepatic artery complications, particularly hepatic artery thrombosis (HAT). The manner by which arterial reconstruction is performed is dependent on the specific circumstances of each child or adolescent.
PATIENT HISTORY AND PHYSICAL FINDINGS
Prior to listing for liver transplantation, the potential recipient must undergo a thorough history and physical examination as well as a full multidisciplinary evaluation. Regarding eventual arterial reconstruction of a liver allograft, younger age and lower recipient weight are factors that contribute to a higher incidence of HAT.1,2
A patient history of thrombosis should initiate a thrombophilia evaluation. A prothrombotic state, such as factor V Leiden mutation, is a risk factor for HAT in liver transplantation.3 A family history of thrombophilia should also be fully explored.
IMAGING AND OTHER DIAGNOSTIC STUDIES
All children undergo a magnetic resonance imaging (MRI) of the abdomen with intravenous contrast to examine the recipient vasculature and to assess for any intraabdominal anomalies, such as those associated with the biliary atresia splenic malformation syndrome. A diminutive recipient hepatic artery and/or the presence of variant hepatic artery anatomy may warrant creation of an aortic conduit.
MRI also allows preoperative assessment of the size of the recipient spleen. Massive splenomegaly is a risk factor for splenic artery steal syndrome and hepatic artery hypoperfusion (and possibly thrombosis).4,5 In the recipient with massive splenomegaly, the surgeon should be prepared for alternative arterial reconstruction options.
SURGICAL MANAGEMENT
Back-Table Preparation of Donor Artery
Place stay sutures (3-0 silk) on the aortic cuff of the celiac axis to allow for manipulation of the artery with minimal forceps handling (FIG 1). Crush injury to the hepatic artery can result in intimal dissection or bleeding in the adventitia. Examine the arterial blood supply for unexpected injuries related to organ procurement and for the presence of anatomic variations, such as a replaced or accessory left or right hepatic artery. Perform dissection of the celiac axis and the common hepatic artery from the aortic cuff to the gastroduodenal artery, sharply clearing excess periadventitial tissue. Ligate branches not going to the liver with fine silk ties or oversew them with 6-0 Prolene suture.
An accessory or replaced right hepatic artery arising from the superior mesenteric artery requires reconstruction to either the splenic artery stump or the gastroduodenal artery stump. Alternative reconstructions include anastomosis of the common hepatic artery to the stump of the superior mesenteric artery or the use of a Y-graft interposition using donor iliac artery. Avoid twisting the accessory or replaced right hepatic artery during this reconstruction.
Intraoperative Decision Making
The most commonly used surgical options for arterial reconstruction in children are primary arterial anastomosis, direct anastomosis of celiac axis to the infrarenal aorta, and creation of an aortic conduit (infrarenal or supraceliac). The decision of which option depends on findings during the transplant operation.
The preferred approach is the use of recipient right and left hepatic arteries as a branch patch for reconstruction with a primary arterial anastomosis. Alternatively, the proper hepatic artery and the gastroduodenal artery may be converted into a branch patch for use as inflow.
Robust pulsatile arterial flow from the recipient arterial system must be confirmed prior to proceeding with a primary anastomosis.
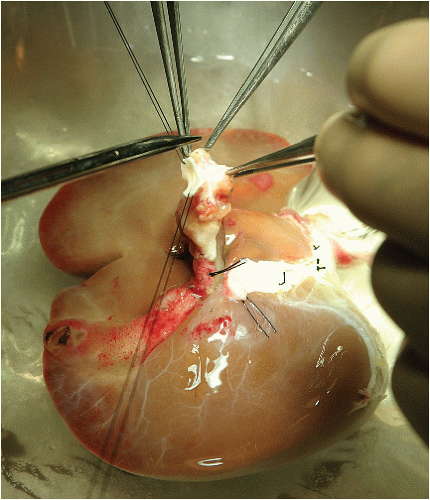
FIG 1 • Back-table preparation of allograft. Stay sutures are placed on the aortic cuff of the celiac axis to allow manipulation with little forceps handling.
In small pediatric recipients (<15 kg) or when the recipient has accessory hepatic arteries, the recipient hepatic artery may be too small to use for inflow. In patients with primary sclerosing cholangitis or primary biliary cirrhosis, the recipient hepatic artery is often extremely delicate and prone to intimal dissection, and therefore, may not be suitable for primary anastomosis.
If the recipient hepatic artery is not suitable for primary anastomosis, place an infrarenal aortic conduit using donor iliac artery as arterial inflow. Dissection of the infrarenal aorta increases the risk of bleeding from retroperitoneal varices. If portal hypertension is severe and a retroperitoneal dissection is hazardous, the aortic conduit can be placed in the supraceliac position.
In some cases, such as split-liver or reduced-size left lateral section allografts, the celiac axis may reach the infrarenal aorta for direct anastomosis, obviating the need for a conduit.
Perform arterial anastomoses under surgical loupe magnification (3.5×). The living donor arterial anastomosis is performed under the operating microscope.
TECHNIQUES
PRIMARY ARTERIAL ANASTOMOSIS
Preparation of Recipient Hepatic Artery and Donor Celiac Axis
Following vena cava and portal venous reconstruction and reperfusion of the allograft, place a vascular clamp on the recipient hepatic artery for inflow control. Remove ties from the divided ends of the right and left hepatic artery branches, and confirm the robustness of the arterial inflow. Flush the recipient artery with heparinized saline solution, and clear the periadventitial tissue. Create a recipient hepatic artery branch patch from the right and left hepatic artery branches (FIG 2). Clear the adventitia at the vessel edges to clearly expose the vessel wall for anastomosis.
Fashion a Carrel patch on the donor celiac axis aortic cuff in order to avoid impinging on the lumen of the vessel during the anastomosis. Alternatively, if caliber matching with the recipient hepatic artery branch patch dictates, excise the aortic cuff to allow anastomosis directly to the orifice of the celiac axis. Flush the donor hepatic artery with heparinized saline solution, and place a microvascular clamp to prevent back-bleeding.
Anastomotic Technique
It is critical to avoid twisting of either the recipient or the donor artery. A caliber-matched, tension-free anastomosis is necessary. Avoid excessive redundancy; it can cause kinking of the artery and result in thrombosis. In order to reduce redundancy, the donor artery can be shortened with the anastomosis to the common hepatic artery. Alternatively, the recipient artery can be shortened with anastomosis to its proper hepatic artery or to a gastroduodenal artery/proper hepatic artery branch patch.
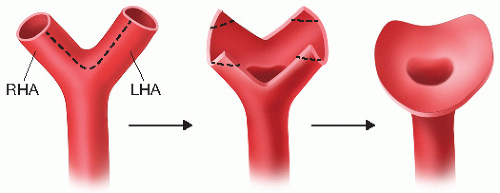
FIG 2 • Creation of native hepatic artery branch patch from right hepatic artery (RHA) and left hepatic artery (LHA).
Place corner sutures transmurally at 3 o’clock and 9 o’clock positions using 6-0 Prolene (FIG 3A). Tie the corner suture opposite the surgeon (3 o’clock position); leave the other corner suture (9 o’clock position) untied to allow the back wall of the anastomosis to be completed more easily.
Beginning at 3 o’clock position, use the 6-0 Prolene suture in a “back wall from inside” technique to complete the back wall of the anastomosis in a running fashion (FIG 3A). Tie 9 o’clock corner suture to itself and then tie the back-wall running suture to this corner suture (FIG 3B). As an alternative to the back wall from inside technique, if the anastomosis can be easily rotated 180 degrees after initial placement of the two corner sutures, the back wall can be exposed as a front wall and completed in a running fashion.
Complete the front wall of the anastomosis by running a 6-0 Prolene suture from the 3 o’clock corner toward the 9 o’clock corner. Tie this suture to the 9 o’clock corner suture to complete the primary arterial anastomosis. If the anastomosis had been rotated 180 degrees to complete the back wall as a front wall, rotate the anastomosis back and complete the front wall.
If the anastomotic diameter is of small caliber (<4 mm), use an interrupted technique for one or both walls of the anastomosis with 7-0 Prolene suture. If there is ample length, rotate the anastomosis 180 degrees to convert the back wall to a front wall.
After completion of the anastomosis, first remove the microvascular clamp distal to the anastomosis followed by the proximal clamp. Inspect the anastomosis and place additional interrupted sutures (7-0 Prolene) to repair leaks. If blood is squirting out from the anastomosis, it will not seal with pressure alone and requires a repair suture. Repair sutures are best performed without obtaining proximal control of the recipient hepatic artery to prevent a back-walled suture. Use Doppler ultrasound to assess flow in the artery and graft. Temporarily clamping the portal vein will facilitate hearing arterial waveforms in the graft.
DIRECT INFRARENAL AORTIC ANASTOMOSIS
Dissection and Preparation of Infrarenal Aorta
If the recipient hepatic artery is not suitable for primary anastomosis, an anastomosis between the donor celiac and the infrarenal aorta can be used if the donor artery is long enough. Dissect the infrarenal aorta prior to removal of the recipient liver. Medially mobilize the right colon and the duodenum to expose the vena cava and the infrarenal aorta. Expose the infrarenal aorta from the left renal vein to the inferior mesenteric artery. This dissection may be difficult due to the presence of retroperitoneal varices. Only dissect as much as you need for placement of a side-biting vascular clamp. It is critical to avoid damage to the recipient renal arteries during this dissection.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree









