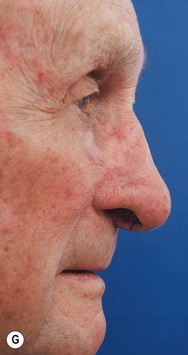
6 Aesthetic nasal reconstruction
Synopsis
 The field of plastic surgery originated with the first early attempts to reconstruct the face, especially the nose.
The field of plastic surgery originated with the first early attempts to reconstruct the face, especially the nose.
 The face tells the world who we are and materially influences what we can become.
The face tells the world who we are and materially influences what we can become.
 The restoration of a normal appearance and an open airway to allow comfortable breathing remains the goal.
The restoration of a normal appearance and an open airway to allow comfortable breathing remains the goal.
 Treatment choices will depend on an understanding of the deformity and wound healing, missing anatomic layers, available donor tissues, surgical planning, the surgeon’s ability to modify them into “like” tissue, and the advantages, disadvantages, and limitations of the technique and its ability to achieve the desired outcome.
Treatment choices will depend on an understanding of the deformity and wound healing, missing anatomic layers, available donor tissues, surgical planning, the surgeon’s ability to modify them into “like” tissue, and the advantages, disadvantages, and limitations of the technique and its ability to achieve the desired outcome.
Historical perspective
The history of facial and nasal reconstruction is the history of plastic surgery.1
Cover
The origins of forehead rhinoplasty (the Indian method) are obscure.2 Nasal repair was described in the Hindu Book of Revelation, the Samhita Susruta, as early as 400 ad and probably was performed before the birth of Christ. Italian surgeons, Branca and Tagliocozzi, reconstructed the nose with skin from the upper arm in the late 1400s.3,4
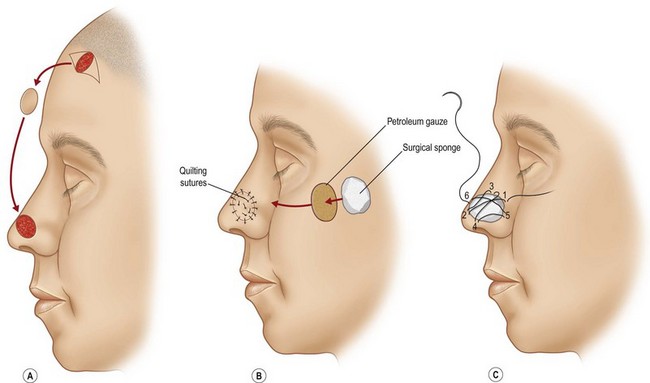
The first written English account of an Indian midline forehead rhinoplasty appeared in the Madras Gazette in 1793 and was republished in the Gentleman’s Magazine of London 1 year later. Carpue,5 an English surgeon, published his account of two successful operations in 1816. This classic vertically oriented median forehead flap design was popularized in the US by Kanzanjian6 in 1946. The base of the flap, which harvested midline forehead tissues and twisted 180°, was supplied by paired supratrochlear vessels. Its pedicle base was located above the eyebrows.
These early repairs were unlined. The external shape of the nose and its airways became distorted by the contracting scar on the underlying raw surface of the external flap.7
The importance of lining replacement did not become clear until the period between 1840 and World War I.8 Because residual intranasal mucosa mucous seemed inadequate, Petralli folded the distal end of the flap on to itself for lining around 1842. However, because of the high pivot point of the classic median flap, its length was inadequate to create a long columella, satisfactory projection, or to permit infolding for lining without transferring hair-bearing skin on the distal end of the flap.
To increase flap length distally, Auvert, in 1850, slanted the flap’s design obliquely across the forehead. German surgeons designed forehead flaps horizontally, supplied by the supraorbital vessels on one side. In 1935, Gillies8 described an up-and-down flap which was centered over one supraorbital pedicle, passed into the hair-bearing scalp, and then descended back into the forehead. In 1942, Converse9,10 modified the up-and-down flap by creating a long pedicle based on the lateral blood supply of the scalp to camouflage scars within the hair-bearing skin. Other designs included New’s sickle flap,11 which transferred skin from the temple recess based on the ipsilateral superficial temporal vessels, or Washio’s flap,12 which transferred skin from behind the ear, based on the anastomosis of the postauricular and superficial temporal vessels.
Unfortunately, these modifications created a forehead defect that was harder to close.
These innovations reduced the twist of the pedicle base and brought the flap closer to the recipient site by lowering its point of rotation. This vertical paramedian flap transferred forehead tissue on a unilateral pedicle blood supply, located near the medial canthus. The anatomic studies of MacCarthy et al.13 and Reece et al.14 demonstrate that the forehead is perfused by an arcade of vessels supplied by the supraorbital, supratrochlear, infraorbital, dorsal nasal, and angular branches of facial artery and on branches of the superficial temporal artery. A rich plexus of vessels, centered on the medial canthus, can reliably perfuse a unilateral flap.
Refinements also developed to minimize potential donor deformity. Velpech, in 1828, designed his flap as a reversed ace of spades, with its stem forming the columella and its tapering tip as the pedicle. Labat, in 1834, designed a similar tripod-shaped flap, with limbs extending obliquely across the forehead. Millard,15 in the 1960s, used a seagull-shaped flap with a central vertical component and lateral wings. The wings extended horizontally, as lateral extensions which resurfaced the ala and flowed into the alar bases and nostril sills. The vertical component covered the dorsum, tip, and columella. Undermining of adjacent wound edges allowed partial closure and a relatively inconspicuous midline T-shaped scar, even in large defects.
Lining
As Harold Gillies stated in 1920,8,16,17 “One is struck chiefly with the lack of appreciation of the need for lining membrane for all mucus-lined cavities.” In actual fact, except that the forehead skin most closely resembles nose skin, the origin of cover is the least important.”
For at least 2000 years, surgeons placed a flap over a full-thickness defect but left its raw undersurface to heal secondarily. Unfortunately, the external shape of the nose and the airways became distorted by contracting scar. In the mid 18th century, Petralli folded the distal end of the flap on itself to form, in a manner of speaking, a tip, ala, and columella. Volkman, in 1873, turned down the scar tissue lying adjacent to the defect, as hingeover flaps. Thiersch transferred flaps from other facial areas in 1879.7 Millard,15 in the 20th century, rolled over bilateral nasolabial flaps to line both the alae and columella.
In 1898, Loosen applied a skin graft to the underlying raw surface of the covering flap at flap transfer. However the “take” was inconsistent and late contracture common. Others placed a split- or full-thickness skin graft on the deep surface of the forehead flap during a preliminary operation. Weeks later, once the viability of the graft was assured, the skin-grafted flap was transferred to supply both cover and lining. Gillies,18 in 1943, popularized prelaminating the forehead flap with composite chondrocutaneous grafts. In 1956, Converse19,20 recommended septal mucoperichondrial cartilage grafts. However, these methods of prelamination (formerly referred to as prefabrication) delayed formal repair and created a relatively unsupported and shapeless nose.
Gillies21 developed the skin graft inlay method for the saddle-nose deformity of syphilis and leprosy. If lining and support were lost but the overlying nasal skin remained intact, he released scar on the undersurface of the external skin and applied a skin graft to the underlying raw surface. A permanent internal prosthesis was worn to splint the graft and maintain nasal shape and airway patency.
Burget and Menick,22 realizing that the forehead is composed of skin, subcutaneous fat, and underlying frontalis muscle, tunneled a cartilage graft within a vascularized pocket within the subcutaneous fat of a full-thickness forehead flap. The deep undersurface of the frontalis muscle was skin-grafted for lining. The buried cartilage graft “stented” the skin graft lining, much like Gillies’ external splint.
Burget and Menick23 popularized the use of residual intranasal lining flaps, based on axial vessels, for lateral, heminasal, and near-total nasal defects. Because such intranasal lining flaps were thin and relatively reliable, primary cartilage grafts could be employed simultaneously to build a delicate hard-tissue layer to support and shape the nose during the initial stages of reconstruction.
Menick24,25 modified the traditional folded-flap and lining skin graft approaches. Because the distal end of a folded forehead flap or a lining skin graft heal to the adjacent residual nasal lining within 3–4 weeks, they are no longer dependent on the flap’s pedicle for survival. The distal end of a folded flap (cut free from the proximal flap) or a skin graft (which was initially revascularized from the forehead flap’s deep surface) survives, even when the covering flap is completely re-elevated. This permits the placement of a delayed primary support framework over the newly vascularized lining, prior to pedicle division.
Over the last decade, distant tissue has been transferred for lining, as a free flap. Until recently, the technical elegance of microsurgery has not matched the artistry required to make a normal nose. However, recent advancements by Burget and Walton,26 employing multiple individual forearm skin paddles and a two-stage forehead flap with an intermediate to debulk the mid-vault, and Menick and Salibian’s folded single-paddle radial forearm flap,27 combined with a three-stage forehead flap, have produced good results.
Support
Realizing that a complete cartilage and bone framework must be placed early to support, shape, and brace the repair, Burget and Menick22,23 combined primary cartilage grafts with thin, vascular intranasal flaps. Menick25 recommended a three-stage full-thickness forehead flap approach, combining primary and delayed primary cartilage grafts and staged soft-tissue debulking during an intermediate operation, prior to pedicle division.
Basic science/disease process
Nasal deformity may follow congenital malformation, trauma (including burns), the sequela of skin cancer treatment by excision or radiation, infection, or immune disease.25 Infection must be controlled, tumor eradicated, and immune disease in remission. Often reconstruction is delayed for weeks to years to allow wound stabilization and maturation and verification of disease control.
Planning an aesthetic nasal reconstruction
The traditional approach
Traditionally,22,25 surgeons sought to “fill the hole” and obtain a healed wound. The defect determined the repair. The design and dimension of a skin graft or flap were determined by the apparent, but often distorted, defect. Scars and additional donor injury were overriding concerns. A comprehensive plan to restore multiple, independent, three-dimensional facial features was rarely envisioned. The emphasis was on tissue transfer (skin graft or flap), blood supply, or the replacement of anatomic layers (cover, lining, support). Without primary support placement, unchecked forces of healing led to contracted scars, pincushioning, and airway collapse.
False principles
The presence and number of scars determine the final result: place incisions in existing scars, minimize scars, fear scars
Place a supportive framework and debulk excess tissue secondarily after the soft tissues have healed and matured
The modern approach to nasal reconstruction
Aesthetic results depend upon the surgeon’s and patient’s choices.22,25 The modern approach relies on the visualization of the “normal” and the determination of what is missing, both anatomically and aesthetically.28 This regional unit approach emphasizes the judicious choice and modification of recipient and donor tissues to provide for the exact replacement of facial units. The principles of facial reconstruction have switched from traditional wound perspective (how big, how deep, anatomy, and flap blood supply) to a visual one. A healed wound, tissue survival, or the replacement of anatomic layers are necessary, but not sufficient, to restore a normal appearance and function. The mature surgeon, with training and experience, “sees the future.” He or she conceptualizes what will work among available options, while visualizing the desired result. A plan is formulated, principles outlined, and techniques and methods chosen.
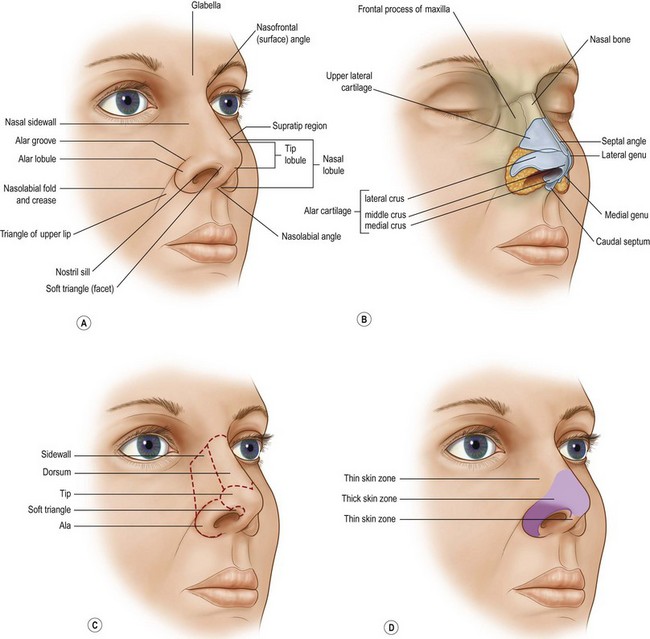
Fortunately, although each defect is different, all repairs are simplified because the “normal” is unchanging. Often, the contralateral normal remains as a visual standard for comparison. If not, the ideal is the guide. The “normal” nose is visually defined by its dimension, volume, position, projection, platform, symmetry, and expected skin quality, border outline, and three-dimensional contour. Major facial landmarks are described as regional units – adjacent topographic areas of characteristic quality, outline, and three-dimensional contour. A unit approach helps the surgeon conceptualize the goal, define the requirements of repair, balance options, and measure the success of the result. Goals, priorities, stages, materials, and method of tissue transfer are clearer with the ideal normal in mind (Fig. 6.1 and Table 6.1).
Table 6.1 Gillies’ and Millard’s “10 commandments” of plastic surgery
(Reproduced from Gillies HD, Millard DR. The principles and art of plastic surgery. Boston: Little Brown, 1957.)
In the latter half of the 20th century, Gonzalez-Ulloa et al.29 divided the face into regions, based on skin thickness. Millard30 envisioned major facial landmarks as “units” and recommended replacing them in their entirety with “like” tissue, of similar color and texture, to avoid a patch-like repair.
Burget and Menick31 divided the nose into “subunits” based on skin quality, border outline, and three-dimensional contour.
The concept of peripheral and central facial units
The face can be divided into areas of characteristic skin quality, border outline, and three-dimensional contour. It can be divided into peripheral and central units. Practically speaking, regional unit concepts provide a rational explanation for clinical observation and treatment recommendations.22,25
Principles of regional unit repair25,28
• Human beings wish to look normal.
• The normal is defined by three-dimensional contour, border outline, and skin quality which describe regional units. They do not correlate with wrinkle or resting skin tension lines.
• The nasal unit consists of the tip, dorsum, columella, and paired alae, sidewalls, and soft triangle subunits.
• Restore units, do not fill defects.
• If a defect, within part of a central unit, is filled without regard to the unit outline, the tissue replacement may appear as a distracting patch within the subunit. The reconstructive goal must restore the character of the unit, rather than simply fill the “hole.”
• Alter the wound in site, size, outline, and depth. Discard adjacent normal tissue within convex nasal subunits to improve the result.
The “subunit principle”22,31
The consequences of the subunit principle are:
1. The defect may be enlarged and the donor requirement increased. A larger defect may preclude closure with local tissue and necessitate a regional flap repair.
2. The number of stages and the complexity of the repair may be increased.
3. The amount of cartilage material required to support the soft tissues may be increased.
4. Patient morbidity may increase if regional flaps or cartilage grafts are needed.
Choose ideal donor materials and employ an ideal method of tissue transfer
Millard’s admonition to use “like for like” applies.30 Use lip for lip, cheek for cheek, and a forehead or nasolabial flap for nasal resurfacing. Distant tissues are employed for lining, to fill dead space, create a facial platform, or vascularize an ischemic, contaminated, or radiated wound. However, distant skin does not match the facial skin quality. Distant skin is not used to resurface the face. Regional skin should be used to replace facial skin.
Classification of defects
The requirements and difficulty of repair are determined by the site, size, and depth of injury. Nasal defects are classified into small, superficial, large, deep, or composite defects.22,25
Composite defects
Composite defects32 extend from the nose on to the adjacent cheek and upper lip. These nose, cheek, and lip units differ visually, anatomically, and functionally. The quality, outline, and contour are different for each facial unit. And the nose sits on the midface cheek and lip platform with characteristic projection, position, and angled relationships.
Treatment/surgical technique and postoperative care
Restoring nasal cover
Skin grafting
Skin grafts have many advantages.22,25 No new scars are added to the nasal surface and the amount of locally available excess tissue is not a limiting factor. Skin grafts are typically harvested from preauricular, postauricular, or supraclavicular donor sites.
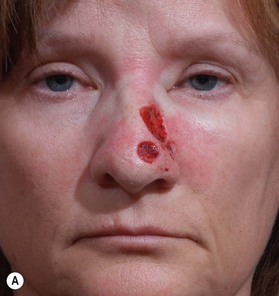
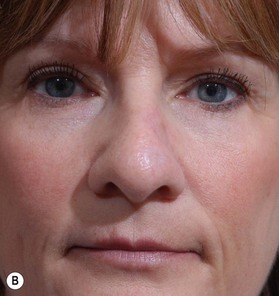
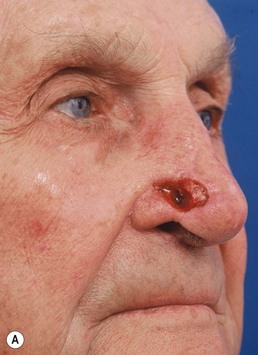
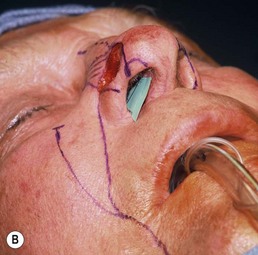
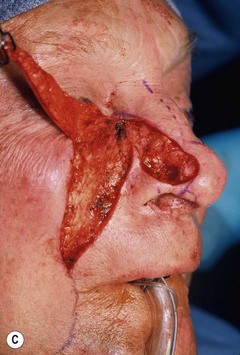
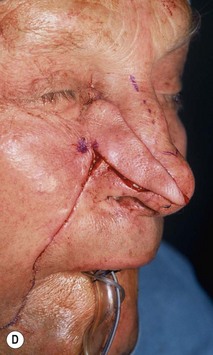
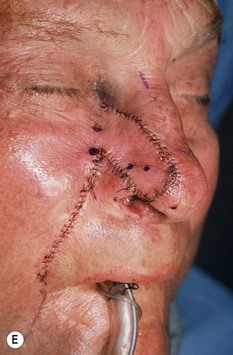
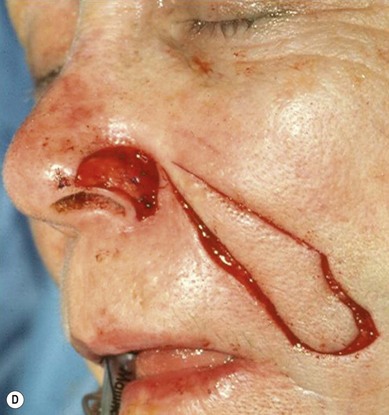
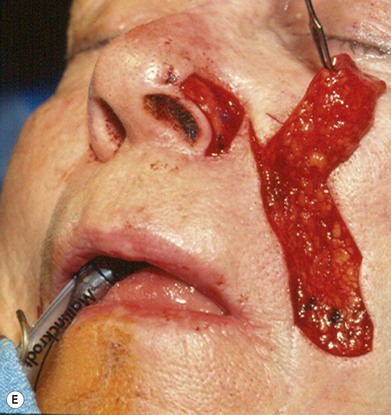
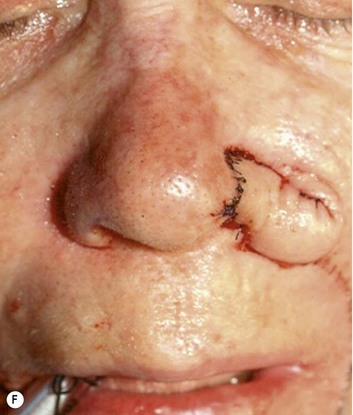
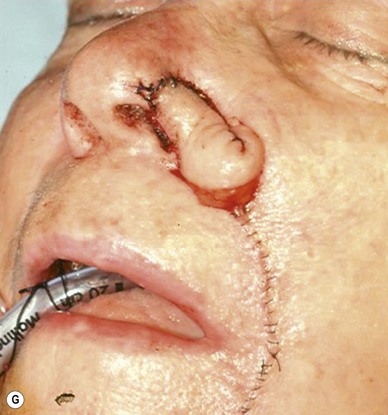
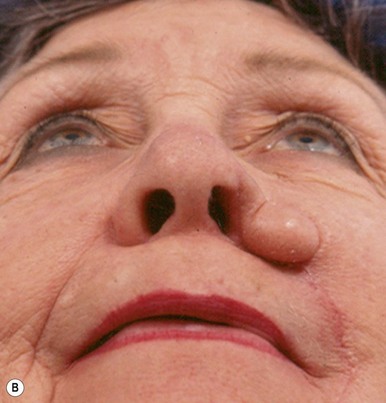
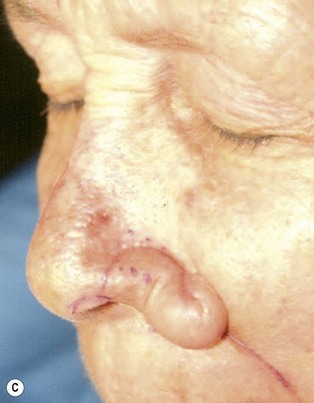
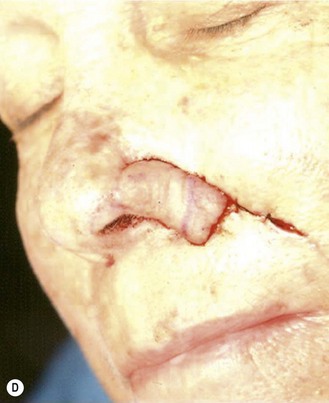
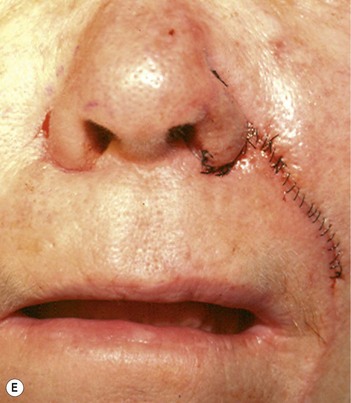
Full-thickness forehead skin graft (Figs 6.2–6.6)
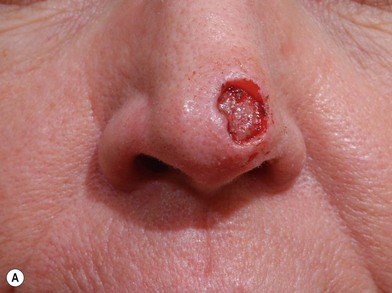
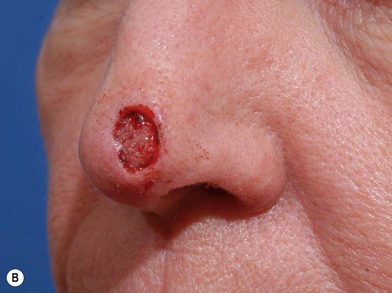
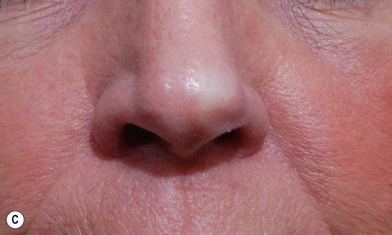
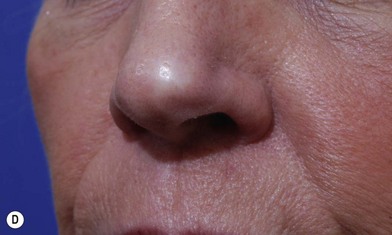
Fig. 6.6 (A–D) Small superficial nasal tip defect resurfaced with full-thickness forehead skin graft.
Local flaps
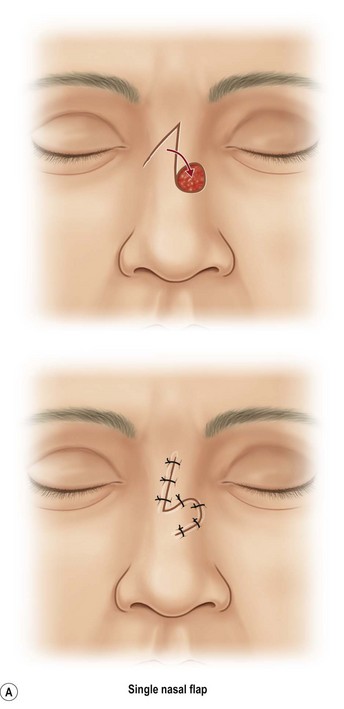
The single-lobe transposition flap (Fig. 6.7)
A modest amount of excess mobile and lax skin is available within the superior nose. A single-lobe transposition flap, designed as a Banner or Romberg flap, is useful for small open defects.33,34 These flaps transpose skin through an arc of 90°, taking excess from one axis to fill a deficiency in another. Because the skin in the superior nose is relatively mobile, available, and lies at a distance from the nostril margins, these small local flaps are unlikely to distort the tip or alar margin. However, if the flap donor scar crosses the bridge transversely, a depressed scar may become visible on profile view. Single-lobed flaps are not useful in the inflexible thick skin of the tip or ala, which shifts poorly. Deforming dog-ears or displacement of adjacent mobile landmarks are common.
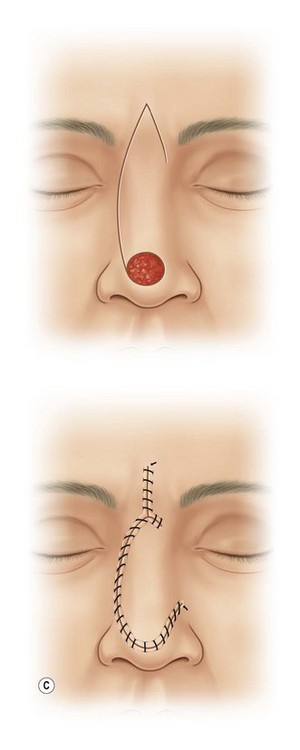
The dorsal nasal flap
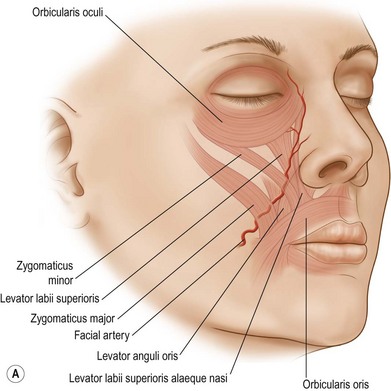
The dorsal nasal flap elevates skin, subcutaneous tissue, and muscle just above the perichondrium and periosteum and slides excess from the glabella towards the tip. The flap is best applied to defects within the dorsum and superior tip subunit.35–37 It is vascularized from facial and angular vessels along the sidewall and medial canthus. Closure is facilitated by advancing cheek skin upward on to the nasal sidewall. The dorsal nasal flap can resurface the nasal tip and dorsum and parts of the ala or sidewall with local skin. Unfortunately, it slides thicker glabellar skin and soft tissue downward on to the nasal sidewall near the medial canthus where a mismatch in skin thickness may create an iatrogenic epicanthal fold. The depth of the radix may be obliterated, effacing the nasal root. Recent modifications have eliminated the glabellar extension. As the dorsal flap slides caudally, a dog-ear is excised inferiorly. Ideally, its borders are planned to lie along the dorsal sidewall junction. The inferior aspect of the flap’s border may be visible as a depressed scar crossing the smooth surface of the tip unit. Like all local flaps, the larger the defect and the closer it lies to the nostril margin, the more likely the tip or rim will be distorted by tension or displaced by poor design.
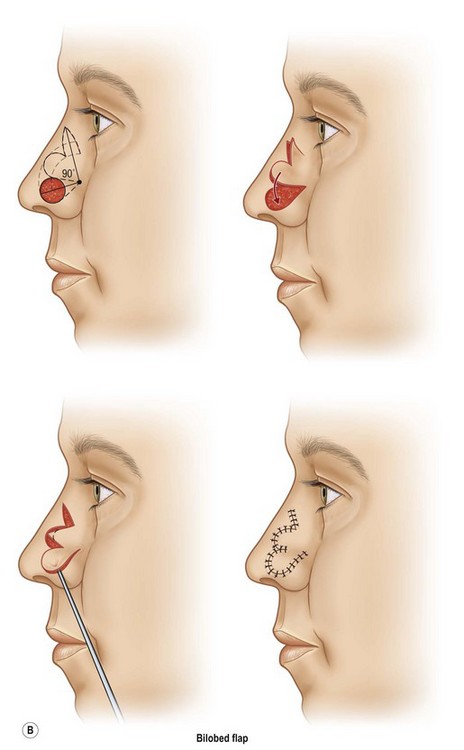
The geometric bilobed flap
The bilobed flap is recommended for defects within the thick, stiff skin of the inferior nose.
The bilobed principle is applied.38–40 Skin is shifted from an area of excess to an area of deficiency by designing the first lobe adjacent to the tip or alar defect. A second lobe, which lies at a distance in an area of tissue availability within the upper nose, is outlined in continuity. As the primary lobe shifts to repair the defect, the secondary lobe resurfaces the defect from the primary lobe. The tertiary defect from the second lobe is closed primarily, within the area of excess.
Traditionally, bilobed flaps were designed with 180° rotation. This created large dog-ears. The dog-ear excision narrowed the flap’s vascular base, jeopardizing blood supply. McGregor and Soutar,39 and later Zitelli,40 developed a geometric design to decrease the flap’s rotation to 90–100° and incorporated a dog-ear excision which did not diminish blood supply. It is useful for defects measuring 0.5–1.5 cm in the inferior nose.
1. The pivot point is established at a distance from the defect equal to one-half of the defect’s diameter (or the radius of the defect). The flap is based laterally for tip defects and medially for defects of the alar lobule. The further the pivot point away from the defect, the larger the flap. Sharing the same pivot point, the circumferences of a larger outer and smaller inner concentric circle are drawn on to the nasal skin. The circumference of the outer circle is outlined at a distance three times the radius of the defect. The second smaller inner circle is marked to equal the distance from the pivot point to the center of the initial defect (the diameter of the defect). Because the nasal surface is round, not flat, a strip of foil or bent paper ruler, rather than a straight ruler, is used as a template which is rotated around the pivot point, like individual spokes of a wheel, until the circumference of both concentric circles is determined.
2. An exact pattern of the circular nasal defect is positioned immediately adjacent to the defect, along the outer concentric circle. The first lobe should replace the defect exactly, to prevent tip or alar rim distortion on closure. Because the second lobe lies within the more mobile skin of the upper nose, it can be designed slightly smaller than the defect. The secondary defect can be partially closed by recruitment of lax adjacent skin within the upper nose. A dog-ear excision, which extends lateral to the outer circle, is added to the second lobe.
3. The dog-ear extending from the defect of the pivot point is excised. The first and second lobes, including the distal dog-ear, are elevated above the periosteum. The flap includes skin, subcutaneous fat, and nasalis muscle. Residual normal nasal skin is undermined widely over the perichondrium and periosteum.
4. Significant pincushioning is infrequent with the bilobed flap, if it is carefully repaired, in layers. The tertiary defect, in the more mobile upper nose, is closed in layers. This pushes the flap inferiorly, preventing the tendency of the flap to return to its donor site. The primary lobe, after appropriate “thinning” so that its skin surface will match the level of the adjacent normal skin, is transferred to the primary defect. The secondary lobe is transposed to fill the gap created by the first. Each flap is fixed in place with sutures to approximate muscle, the subcuticular layer, and skin.
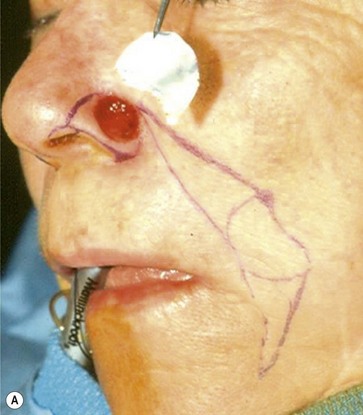
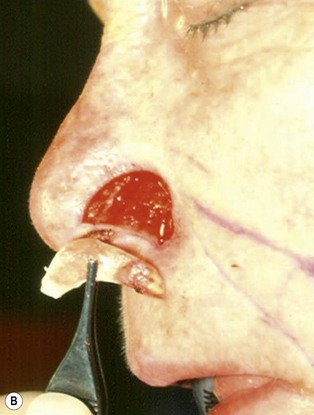
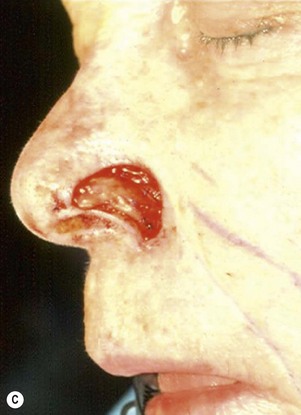
The one-stage nasolabial flap (Fig. 6.8)
The one-stage nasolabial flap can resurface defects of the nasal sidewall and ala, up to 2 cm in size.25 Excess skin of the medial cheek, lateral to the nasolabial fold, is transferred as a random-pattern extension of an advancing cheek flap. Unlike local flaps which redistribute residual nasal skin, this technique “adds” regional cheek skin to the nasal surface. This minimizes the risk of landmark distortion and permits the use of alar support grafts without fear of collapse due to excessive tension.
1. The sidewall and alar subunits are outlined in ink. Alar subunit excision is not performed but skin, which remains between the defect in the inferior nostril rim, can be excised, enlarging the defect inferiorly to the nostril margin. This positions the scar along the nostril border and improves the flap’s blending with the recipient site. The underlying lining is braced with a septal or ear cartilage graft to prevent collapse or retraction of the nostril margin. The ends of the cartilage graft are buried, medially and laterally, in subcutaneous pockets along the rim and at the alar base, and fixed with percutaneous 5-0 polypropylene sutures. The cartilage graft is quilted to the underlying lining to fix the support graft and brace the nostril margin.
2. The nasolabial crease is marked and a pattern of the defect is positioned exactly adjacent to the nasolabial fold so that the late cheek scar lies exactly in the nasolabial crease. A dog-ear excision is marked inferiorly, distal to the template. Ensure that the sliding cheek advancement has adequate length to swing and correctly position the nasolabial extension on to the nasal defect. The most important flap dimension is width, which should equal the width of the defect. The distal margin of the flap will be trimmed during wound closure and does not need to be predetermined. The flap is elevated in continuity with the distal dog-ear. The superior lateral incision for the alar or sidewall flap extension should not extend higher than the alar remnant that must be “jumped over” to reach the recipient site. A higher incision is unnecessary and may impair blood supply.
3. The nasolabial skin extension and the cheek flap are undermined, with a few millimeters of subcutaneous fat, laterally for 3–5 cm. The cheek is advanced, fixing its underlying raw surface to the deep tissues along the nasal facial groove. This suture fixation advances the cheek flap, closes the donor defect, and restores the nasofacial sulcus. It eliminates lateral and vertical tension on the nasolabial extension, which travels with a cheek flap to resurface the primary defect. The vascularity of the random extension is good, but can be impaired by tension.
4. Excess subcutaneous fat is excised to match the thickness of the flap to the depth of the recipient bed. Absorbable sutures can be placed to fix the deep surface of the flap gently to the underlying soft tissues at the ideal alar crease, if vascularity is maintained. If necessary, the crease can be recreated secondarily. The distal flap is gently laid over the inferior aspect of the nasal wound and trimmed to fit. The incisions are closed in layers.
The one-stage nasolabial flap is useful for defects of the sidewall and ala which are not effectively repaired with other local flaps. It can also be used to resurface the upper lip and nasal sill in a composite defect of the nose, lip, and cheek.41–43 It is often combined with the Millard fat flip flap,15,16 which hinges over excess subcutaneous fat, from the lateral cheek, to restore missing premaxillary soft-tissue bulk.
Large, deep, and adversely located defects
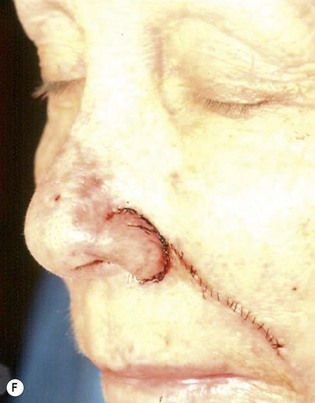
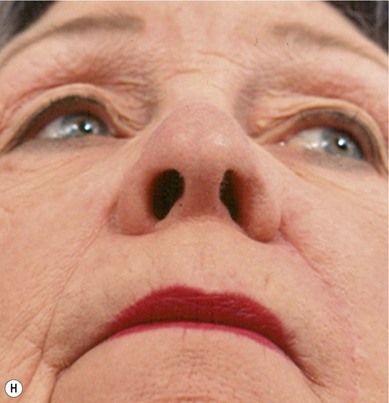
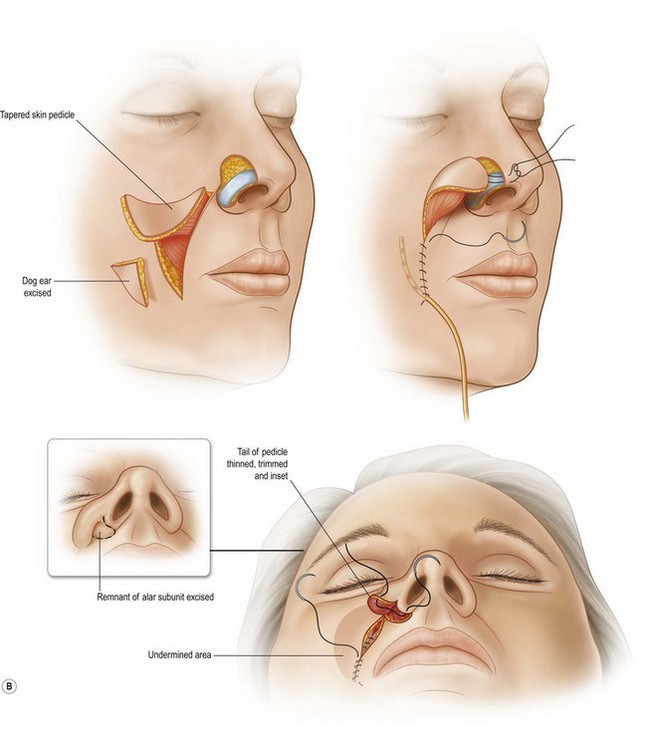
The superiorly based two-stage subunit nasolabial flap (Figs 6.9–6.11)
The two-stage nasolabial flap22,44 transfers excess skin from the medial cheek, just lateral to the nasolabial fold, supplied by blood vessels which originate from the underlying facial and angular arteries, passing through the underlying subcutaneous tissue, above and below the levator labii muscle. Although the narrow skin pedicle contributes a modest random blood supply, the flap is a subcutaneously based island flap. If the underlying subcutaneous vascular base is intact, the flap is reliable even if skin lateral to the ala is scarred or has been excised. The subcutaneous pedicle permits easy transposition and the narrow skin component eliminates the superior dog-ear and its accompanying scar from extending on to the nasal sidewall on wound closure. The flap is designed with its medial border exactly along the nasolabial fold to place the final scar exactly in the nasolabial crease. In young patients or those with a poorly defined fold, the nasolabial crease may be indistinct and should be marked with ink preoperatively, before sedation or general anesthesia.