Acne Vulgaris and Acneiform Eruptions: Introduction
|
Acne Vulgaris
Acne vulgaris is a self-limited disorder of the pilosebaceous unit that is seen primarily in adolescents. Most cases of acne present with a pleomorphic array of lesions, consisting of comedones, papules, pustules, and nodules with varying extent and severity. While the course of acne may be self-limiting, the sequelae can be lifelong, with pitted or hypertrophic scar formation.
Acne is sufficiently common that it often has been termed physiologic. Mild degrees of acne are frequently seen at birth, probably resulting from follicular stimulation by adrenal androgens, and may continue into the neonatal period. However, in the vast majority of cases it is not until puberty that acne becomes a more significant problem. Acne often heralds the onset of puberty. In girls, the occurrence of acne may precede menarche by more than a year. In these very young patients, the predominant lesions are comedones. Acne prevalence hits its peak during the middle-to-late teenage period, with more than 85% of adolescents affected, and then steadily decreases. However, acne may persist through the third decade or even later, particularly in women. One study demonstrated a prevalence of facial acne in women between ages 26 and 44 to be 14%.1 Acne severity seems to be familial. The prevalence of high school students with moderate-to-severe acne was 19.9% in those students with a family history of acne and 9.8% in those students without a family history of acne.2 In twin studies, 81% of the population variance in acne was found due to genetic factors (vs. 19% environmental factors).3 Nodulocystic acne has been reported to be more common in white males than in black males, and one group of investigators has found that acne is more severe in patients with the XYY genotype.4,5
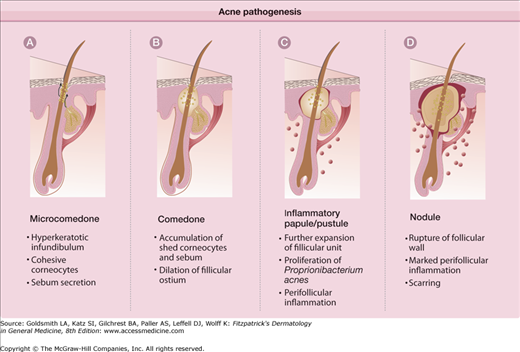
Understanding the underlying basis for acne, and the mechanisms of action of the multitude of therapeutic options in treating acne will assure better therapeutic results. The pathogenesis of acne is multifaceted, but four basic steps have been identified. These key elements (Fig. 80-1) are: (1) follicular epidermal hyperproliferation, (2) excess sebum production, (3) inflammation, and (4) the presence and activity of Propionibacterium acnes. Each of these processes are interrelated and under hormonal and immune influence.
Follicular epidermal hyperproliferation results in the formation of a microcomedo. The epithelium of the upper hair follicle, the infundibulum, becomes hyperkeratotic with increased cohesion of the keratinocytes. The excess cells and their tackiness result in a plug in the follicular ostium. This plug then causes downstream concretions of keratin, sebum, and bacteria to accumulate in the follicle. These packed concretions cause dilation of the upper hair follicle producing a microcomedo.
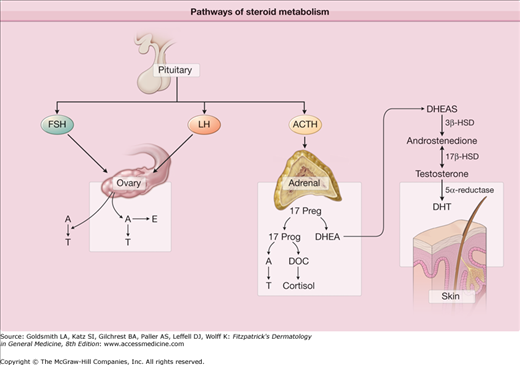
The stimulus for keratinocyte hyperproliferation and increased adhesion is unknown. However, several proposed factors in keratinocyte hyperproliferation include: androgen stimulation, decreased linoleic acid, increased interleukin-1 (IL-1) α activity, and effects of P. acnes. Dihydrotestosterone (DHT) is a potent androgen that may play a role in acne. Fig. 80-2 demonstrates the physiologic pathway for dehydroepiandrosterone sulfate (DHEA-S) conversion to the androgen DHT. 17-β hydroxysteroid dehydrogenase (HSD) and 5-α reductase are enzymes responsible for converting DHEA-S to DHT. When compared to epidermal keratinocytes, follicular keratinocytes have increased 17-β HSD and 5-α reductase, thus enhancing DHT production.6,7 DHT may stimulate follicular keratinocyte proliferation. Also supporting the role of androgens in acne pathogenesis is the evidence that individuals with complete androgen insensitivity do not develop acne.8 Follicular keratinocyte proliferation may also be regulated by linoleic acid. Linoleic acid is an essential fatty acid in the skin that is decreased in subjects with acne. The quantity of linoleic acid normalizes after successful treatment with isotretinoin. Subnormal levels of linoleic acid may induce follicular keratinocyte hyperproliferation and produce proinflammatory cytokines. It has also been suggested that regular quantities of linoleic acid are actually produced but are simply diluted by increased sebum production.9 In addition to androgens and linoleic acid, IL-1 α may also contribute to keratinocyte hyperproliferation. Human follicular keratinocytes demonstrate hyperproliferation and microcomedone formation when IL-1 α is added. IL-1 receptor antagonists inhibit microcomedone formation providing additional support for the cytokine’s role in acne pathogenesis.10,11 Fibroblast growth factor receptor (FGFR)-2 signaling may also be involved in hyperkeratinization. There is a long established relationship between acne and Apert syndrome, a complex bony malformation syndrome, due to a gain in function mutation in the gene encoding FGFR-2. Mutations in FGFR-2 in a mosaic distribution underlie a nevus comedonicus-like lesion.12 The FGFR-2 pathway is androgen dependent and proposed mechanisms in acne include an increased production of IL-1 α and 5-α reductase.13,14
Figure 80-2
Pathways of steroid metabolism. Dehydroepiandrosterone (DHEA) is a weak androgen that is converted to the more potent testosterone by 3β-hydroxysteroid dehydrogenase (HSD) and 17β-HSD. 5-α reductase then converts testosterone to dihydrotestosterone (DHT), the predominant hormonal effector on the sebaceous gland. The sebaceous gland expresses each of these enzymes. A = androstenedione; ACTH = adrenocorticotropin-stimulating hormone; DHEAS = dehydroepiandrosterone sulfate; E = estrogen; FSH = follicle-stimulating hormone; LH = luteinizing hormone; T = testosterone; DOC = deoxycortisol.
The second key feature in the pathogenesis of acne is excess sebum production from the sebaceous gland. Patients with acne produce more sebum than those without acne, although the quality of sebum is the same between the two groups.15 Components of sebum—triglycerides and lipoperoxides—may play a role in acne pathogenesis. Triglycerides are broken down into free fatty acids by P. acnes, normal flora of the pilosebaceous unit. These free fatty acids promote further bacterial clumping and colonization of P. acnes, incite inflammation, and may be comedogenic.16 Lipoperoxides also produce proinflammatory cytokines and activate the peroxisome proliferator-activated receptors (PPAR) pathway, resulting in increased sebum.17,18
Androgenic hormones also influence sebum production through actions on sebocyte proliferation and differentiation. Similar to their action on the follicular infundibular keratinocytes, androgen hormones bind to and influence sebocyte activity.19 Those with acne have higher average serum androgen levels (although still within normal range) than unaffected controls.20,21 5-α reductase, the enzyme responsible for converting testosterone to the potent DHT, has greatest activity in areas of skin prone to acne, the face chest and back.14
The role of estrogen on sebum production is not well defined. The dose of estrogen required to decrease sebum production is greater than the dose required to inhibit ovulation.22 The mechanisms by which estrogens may work include: (1) directly opposing the effects of androgens within the sebaceous gland; (2) inhibiting the production of androgens by gonadal tissue via a negative feedback loop on pituitary gonadotropin release; and (3) regulating genes that suppress sebaceous gland growth or lipid production.23
Corticotropin-releasing hormone may also play a role. It is released by the hypothalamus and increased in response to stress. Corticotropin-releasing hormone receptors are present on a vast number of cells, including keratinocytes and sebocytes, and are upregulated in the sebocytes of patients with acne.24
The microcomedo will continue to expand with densely packed keratin, sebum, and bacteria. Eventually this distension will cause follicular wall rupture. The extrusion of the keratin, sebum, and bacteria into the dermis results in a brisk inflammatory response. The predominant cell type within 24 hours of comedo rupture is the lymphocyte. CD4+ lymphocytes are found around the pilosebaceous unit, while CD8+ cells are found perivascularly. One to two days after comedo rupture, the neutrophil becomes the predominant cell type surrounding the burst microcomedo.25
It was originally thought that inflammation follows comedo formation, but there is evidence that dermal inflammation may actually precede comedo formation. Biopsies taken from comedo-free acne-prone skin, demonstrate increased dermal inflammation compared to normal skin. Biopsies of newly formed comedos demonstrate even greater inflammation.26 This may suggest that inflammation actually precedes comedo formation, again emphasizing the interplay between all of the pathogenic factors. As mentioned above, P. acnes also plays an active role in the process of inflammation. P. acnes is a Gram-positive, anaerobic, and microaerobic bacterium found in the sebaceous follicle. Adolescents with acne have higher concentrations of P. acnes compared to nonacne controls. However, there is no correlation between the raw number of P. acnes organisms present in a sebaceous follicle and the severity of the acne.27 Sebocyte differentiation and proinflammatory cytokine/chemokine responses are varied depending on the strain of P. acnes predominating within the follicle.28
The cell wall of P. acnes contains a carbohydrate antigen that stimulates antibody development. Those patients with the most severe acne have the highest titers of antibodies.29 The antipropionobacterium antibody enhances the inflammatory response by activating complement initiating a cascade of proinflammatory events.30 P. acnes also facilitates inflammation by eliciting a delayed type hypersensitivity response and by producing lipases, proteases, hyaluronidases, and chemotactic factors.31,32 Reactive oxygen species and lysosomal enzymes are released by neutrophils and levels may correlate with severity.33 Additionally, P. acnes has been shown to stimulate expression of cytokines by binding to toll-like receptor 2 (TLR-2) on monocytes and polymorphonuclear cells surrounding the sebaceous follicle.34 After binding TLR-2, proinflammatory cytokines such as IL-1α, IL-8, IL-12, and TNF-α are released.35,36 The antimicrobial peptides, histone H4 and cathelicidin, are also secreted locally in response to P. acnes. Histone H4 exerts direct microbial killing, while cathelicidin interacts with components of the innate immune system, such as β defensins and psoriasin, in response to P. acnes.37,38 Another indicator of the role of innate immunity in the pathogenesis of acne is the differentiation of peripheral blood monocytes to CD209+ macrophages and CD1b+ dendritic cells in response to P. acnes.39
The impact of diet on acne is an emerging area of interest, particularly relating to glycemic index and dairy consumption. Both are thought to increase insulin-like growth factor (IGF)-1 with possible proacne effects and an increase in androgen activity.40,41
Most patients with acne vulgaris report gradual onset of lesions around puberty. In other cases, acne can be seen in the neonatal or infantile age. Neonatal acne appears at about 2 weeks of age and infantile acne develops at 3–6 months of age (see Chapter 107). Since classic acne vulgaris is usually gradual in onset, patients describing an abrupt onset of acne should be questioned to possibly discover an underlying etiology, such as an androgen-secreting tumor.
Hyperandrogenism should be considered in the female patient whose acne is severe, sudden in its onset, or associated with hirsutism or irregular menstrual periods. The patient should be asked about the frequency and character of her menstrual periods and whether her acne flares with changes in her menstrual cycle. Hyperandrogenism can also result in deepening of the voice, an increase in libido and hirsutism. A complete medication history is important, as some medications can cause an abrupt onset of a monomorphous acneiform eruption. Drug-induced acne may be caused by: anabolic steroids, corticosteroids, corticotropin, phenytoin, lithium, isoniazid, vitamin B complexes, halogenated compounds, and certain chemotherapy medications, particularly with epidermal growth factor receptor (EGFR) inhibitors.
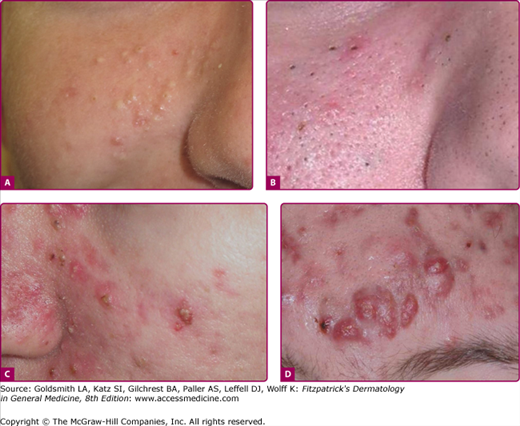
The primary site of acne is the face and to a lesser degree the back, chest, and shoulders. On the trunk, lesions tend to be concentrated near the midline. The disease is characterized by several clinical lesion types (Fig. 80-3). Although one type of lesion may predominate, close inspection usually reveals the presence of several types of lesions. The lesions may be either noninflammatory or inflammatory. The noninflammatory lesions are comedos, which may be either closed (whiteheads; Fig. 80-3A) or open (blackheads; Fig. 80-3B). The open comedo appears as a flat or slightly raised lesion with a central dark-colored follicular impaction of keratin and lipid (Fig. 80-4). Closed comedones, in contrast to the open comedones, may be difficult to visualize. They appear as pale, slightly elevated, small papules, and do not have a clinically visible orifice (Fig. 80-3A). Stretching of the skin is an aid in detecting the lesions.
Figure 80-3
Clinicopathologic correlation of acne lesions. A. Closed comedone. The follicular infundibulum is distended, filled with keratin and sebum, and the follicular epithelium is attenuated. The follicular ostium is narrow. B. Open comedone. Resembles the closed comedone with the exception of a patulous follicular ostium. C. Inflammatory papule. Acute and chronic inflammatory cells surround and infiltrate the follicle, which shows infundibular hyperkeratosis. D. Nodule. The follicle is filled with acute inflammatory cells. With the rupture of the distended follicle, there is a foreign body granulomatous response.
Figure 80-4
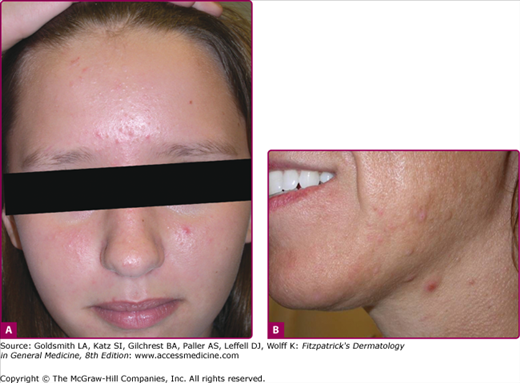
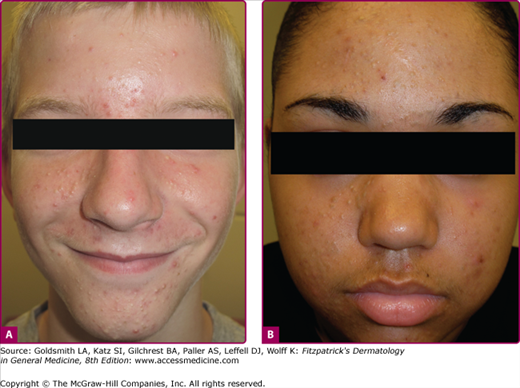
Acne vulgaris mild. A. A 13-year-old girl with mild acne vulgaris. Scattered comedones and/or inflammatory lesions are seen, usually limited to less than half of the face. The T-zone of the face is commonly involved. No nodules are present. B. An adult female with primarily inflammatory acne. Note the typical involvement of the jawline.
The inflammatory lesions vary from small papules with a red border to pustules and large, tender, fluctuant nodules (see Figs. 80-3C and 80-3D and Figs. 80-4, 80-5, and 80-6). Some of the large nodules were previously called “cysts” and the term nodulocystic has been used to describe severe cases of inflammatory acne. True cysts are rarely found in acne; this term should be abandoned and substituted with severe nodular acne (see Figs. 80-3D and 80-6). Whether the lesion appears as a papule, pustule, or nodule depends on the extent and location of the inflammatory infiltrate in the dermis.
Figure 80-5
Acne vulgaris moderate. A. A 15-year-old male with moderate acne is seen. Typically more than half of the face is involved with increasing numbers of lesions, usually a mix of lesions is seen: papules, pustules, and comedones. Infrequent and limited nodules may be present. Chest and back involvement may also be moderately affected. B. A 16-year-old female with open and deep closed comedones is pictured. Scarring and postinflammatory changes are possible sequelae.
Figure 80-6
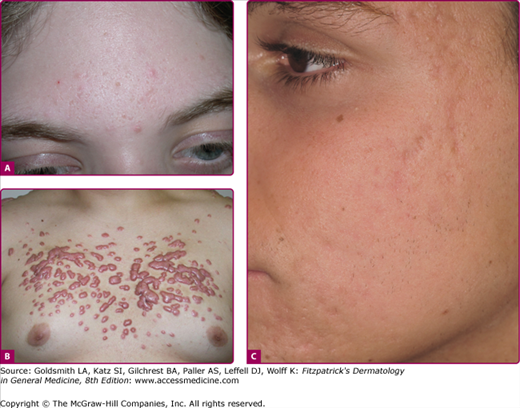
Acne vulgaris severe. A. A 17-year-old female with extensive acne is seen. Numerous pustules and nodular lesions admixed with comedones and smaller papules cover the entire face. B. Deep, friable nodules that coalesce into pseudocysts are seen in acne conglobata. C. Chest and back involvement can be extensive and severe. Scarring is a common complication in severe acne.
Scarring can be a complication of both noninflammatory and inflammatory acne. There are four general types of acne scars: (1) ice pick, (2) rolling, (3) boxcar, and (4) hypertrophic42 (Fig. 80-7). Ice pick scars are narrow, deep scars that are widest at the surface of the skin and taper to a point in the dermis. Rolling scars are shallow, wide scars that have an undulating appearance. Boxcar scars are wide sharply demarcated scars. Unlike ice pick scars, the width of boxcar scars is similar at the surface and base. In rare instances, especially on the trunk, the scars may be hypertrophic.
Acne vulgaris is usually an isolated cutaneous finding, other than in the presence of hyperandrogenism. Such cases may have associated hirsutism, precocious puberty, and other signs of hyperandrogenism.
In general, laboratory workup is not indicated for patients with acne unless hyperandrogenism is suspected. There are numerous clinical studies relating acne to elevated serum levels of androgens in both adolescents and adults. Among 623 prepubertal girls, girls with acne had increased levels of DHEAS as compared to age-matched controls without acne.43 DHEAS can serve as a precursor for testosterone and DHT. Elevated serum levels of androgens have been found in cases of severe cystic acne and in acne associated with a variety of endocrine conditions, including congenital adrenal hyperplasia (11β- and 21β-hydroxylase deficiencies), ovarian or adrenal tumors, and polycystic ovarian disease. However, in the majority of acne patients serum androgens are within the normal range.44,45
Excess androgens may be produced by either the adrenal gland or ovary. The laboratory workup should include measurement of serum DHEAS, total testosterone, and free testosterone. Additional tests to consider include the luteinizing hormone (LH) to follicle-stimulating hormone (FSH) ratio or serum 17-hydroxyprogesterone to identify an adrenal source of androgens in cases where testing does not clearly indicate an adrenal or ovarian source of androgens. Testing should be obtained just prior to or during the menstrual period, not midcycle at the time of ovulation. Patients on contraceptives that prevent ovulation will need to discontinue their medication for at least 1 month prior to testing. Values of DHEAS in the range of 4,000–8,000 ng/mL (units may vary at different laboratories) may be associated with congenital adrenal hyperplasia. Patients with a serum level of DHEAS >8,000 ng/mL could have an adrenal tumor and should be referred to an endocrinologist for further evaluation. An ovarian source of excess androgens can be suspected in cases where the serum total testosterone is >150 ng/dL. Serum total testosterone in the range of 150–200 ng/dL or an increased LH/FSH ratio (>2.0) can be found in cases of polycystic ovary disease. Greater elevations in serum testosterone may indicate an ovarian tumor, and appropriate referral should be made. There is a significant amount of variability in individual serum androgen levels. In cases in which abnormal results are obtained, it may be wise to repeat the test before proceeding with therapy or additional testing.
Many patients report that their acne flares during periods of stress. Although objective data are limited, stress is known to increase the output of adrenal steroids, which may affect the sebaceous gland.46 It has been shown that patients with acne have a greater increase in urinary glucocorticoid levels after corticotropin administration.47
Although one type of lesion may predominate, acne vulgaris is diagnosed by a variety of acne lesions (comedones, pustules, papules, and nodules) on the face, back, or chest (see Box 80-1). Diagnosis is usually easy, but inflammatory acne may be confused with folliculitis, rosacea, or perioral dermatitis. Patients with tuberous sclerosis and facial angiofibromas have been misdiagnosed as having recalcitrant midfacial acne. Facial flat warts or milia are occasionally confused with closed comedones.
Most Likely
|
Consider
|
Always Rule Out
|
Acne can be seen in association with endocrinologic abnormalities. Patients with hyperandrogenism may have acne plus other stigmata of increased androgen levels (i.e., hirsutism, deepened voice, irregular menses). Endocrinologic disorders such as polycystic ovarian syndrome (including HAIR-AN syndrome), congenital adrenal hyperplasia, and adrenal and ovarian neoplasms often have accompanying acne.
Variants of acne must also be differentiated from typical acne vulgaris in order to guide treatment. These types of acne include: neonatal acne, infantile acne, acne fulminans, acne conglobata, acne with solid facial edema, and acne excoriée des jeunes filles. These variants are discussed in detail later in the chapter.
There are several less common acneiform eruptions that can be confused with acne vulgaris. These mimickers include: medication-induced acne, halogen acne, chloracne, acne mechanica, tropical acne, radiation acne, and other various miscellaneous acneiform disorders that are discussed subsequently.
All types of acne lesions have the potential to resolve with sequelae. Almost all acne lesions leave a transient macular erythema after resolution. In darker skin types, postinflammatory hyperpigmentation may persist months after resolution of acne lesions. In some individuals, acne lesions may result in permanent scarring.
Acne vulgaris may also take a psychological toll on many patients. It is estimated that 30%–50% of adolescents experience psychiatric disturbances due to acne.48 Studies have shown that patients with acne have similar levels of social, psychological, and emotional impairment as those with asthma and epilepsy.49 Additional studies have also shown that unemployment rates are higher among adults with acne than those without.50 When appropriate, patients should be referred for psychiatric counseling.
The age of onset of acne varies considerably. It may start as early as 6–8 years of age or it may not appear until the age of 20 or later. The course is one of several years’ duration followed by spontaneous remission in the majority of cases. While most patients will clear by their early twenties, some have acne extending well into the third or fourth decades. The extent of involvement varies, and spontaneous fluctuations in the degree of involvement are the rule rather than the exception. In women there is often a fluctuation in association with menses, with a flare just before the onset of menstruation. This flare is not due to a change in sebaceous gland activity as there is no increase in sebum production in the luteal phase of the menstrual cycle. It has been shown that prepubescent females with comedonal acne and those females with high DHEAS levels are predictors of severe or long-standing nodulocystic acne.51
Tailoring a patient’s acne regimen with the knowledge of the pathogenesis of acne and the mechanism of action of the available acne treatments will ensure maximum therapeutic response. Treatment regimens should be initiated early and be sufficiently aggressive to prevent permanent sequelae. Often multiple treatments are used in combination so as to combat many factors in the pathogenesis of acne (Table 80-1). The mechanism of action of the most common treatments for acne can be categorized in the following categories as they relate to the pathophysiology:
Correct the altered pattern of follicular keratinization.
Decrease sebaceous gland activity.
Decrease the follicular bacterial population, particularly P. acnes.
Exert an anti-inflammatory effect.
Mild | Moderate | Severe | Nodular | Conglobata/Fulminans | |
|---|---|---|---|---|---|
Comedonal | Papular/Pustular | Papular/Pustular | |||
First | Topical retinoid or combinationa | Topical retinoid + topical antimicrobial or combinationa | Oral antibiotic + topical retinoid ± BPO or combinationa | Oral antibiotic + topical retinoid ± BPO | Oral isotretinoin ± oral corticosteroids |
Second | Topical dapsone or azelaic acid or salicylic acid | Topical dapsone or azelaic acid or salicylic acid | Oral antibiotic + topical retinoid ± BPO or combinationa | Oral isotretinoin or oral antibiotic + topical retinoid ± BPO/azelaic acid or combinationa | High-dose oral antibiotic + topical retinoid + BPO or combinationa |
Female | — | — | + Oral contraceptive/antiandrogen | + Oral contraceptive/antiandrogen | + Oral contraceptive/antiandrogen |
Additional options | Comedone extraction | Laser/light therapy, photodynamic therapy | Comedone extraction, laser/light therapy, photodynamic therapy | Comedone extraction; intralesional corticosteroid, laser/light therapy, photodynamic therapy | Intralesional corticosteroid, laser/light therapy, photodynamic therapy |
Refractory to treatment | Check compliance | Check compliance Exclude Gram-negative folliculitis Females: Exclude polycystic ovary syndrome, adrenal or ovarian tumors, congenital adrenal hyperplasia Males: Exclude congenital adrenal hyperplasia | |||
Maintenance | Topical retinoid ± BPO, or combinationa | Topical retinoid ± BPO, or combinationa | Topical retinoid ± BPO, or combinationa | Topical retinoid ± BPO, or combinationa |
The importance of cleansing in the treatment of acne is generally intuitive. Twice daily washing with a gentle cleanser followed by the application of acne treatments may encourage a routine and therefore better compliance. Overcleansing or using harsh alkaline soaps are likely to increase the skin’s pH, disrupt the cutaneous lipid barrier, and compound the irritancy potential of many topical acne treatments. Use of a syndet (synthetic detergent) will allow cleansing without disruption of the skin’s normal pH. Antibacterial soaps, containing agents such as triclosan, inhibit Gram-positive cocci but may increase Gram-negative rods; their overall affect on acne is unclear. Medicated cleansers, containing benzoyl peroxide or salicylic acid, offer convenience as a wash and are excellent for hard to reach areas like the back.
(See Table 80-2)
Generic | Trade | Vehicle | Concentration | Size |
|---|---|---|---|---|
Retinoids—Topical | ||||
Tretinoin | Retin-A | Cream | 0.025%, 0.05%, 0.1% | 20 g, 45 g |
Gel | 0.01%, 0.025% | 15 g, 45 g (0.025% only) | ||
Liquid | 0.05% | 28 mL | ||
Retin-A micro | Gel with microsponge | 0.04%, 0.1% | 20 g, 45 g 50-g pump | |
Avita | Cream | 0.025% | 20 g, 45 g | |
Gel | 0.025% | 20 g, 45 g | ||
Refissa | Cream | 0.05% | 40 g | |
Tretin-X | Cream | 0.025%, 0.05%, 0.1% | 35 g (kit with cleanser) | |
Gel | 0.025%, 0.1% | 35 g (kit with cleanser) | ||
Generic | Cream | 0.025%, 0.05%, 0.1% | 20 g, 45 g | |
Gel | 0.025%, 0.1% | 15 g, 45 g | ||
Adapalene | Differin | Cream | 0.1% | 15 g, 45 g |
Gel | 0.1%, 0.3% | 15 g, 45 g | ||
Lotion | 0.1% | 2 oz | ||
Generic | Gel | 0.1% | 45 g | |
Tazarotene | Tazorac | Cream | 0.1% | 30 g, 60 g |
Gel | 0.1% | 30 g, 60 g | ||
Retinoid Combinations—Topical | ||||
Tretinoin/clindamycin | Ziana | Gel | 0.025%/1.2% | 30 g, 60 g |
Adapalene/benzoyl peroxide | Epiduo | Gel | 2.5%/0.1% | 45 g |
Antimicrobials—Topical | ||||
Benzoyl peroxide | Benzac AC | Gel | 2.5%, 5%, 10% | 60 g |
Wash | 2.5%, 5%, 10% | 240 mL (2.5%), 226 mL | ||
Benzac W | Gel | 2.5%, 5%, 10% | 60 g | |
Wash | 5%, 10% | 226 mL | ||
Benzashave | Cream | 5%, 10% | 113.4 g | |
Benziq LS | Gel | 5.25% | 50 g | |
Wash | 5.25% | 175 g | ||
Brevoxyl | Gel | 4%, 8% | 42.5 g | |
Creamy wash | 4%, 8% | 170 g (kit with cleanser) | ||
Clinac | Gel | 7% | 45 g | |
Desquam E | Gel | 2.5%, 5%, 10% | 42.5 g | |
Triaz | Gel | 3%, 6%, 9% | 42.5 g | |
Cleanser | 3%, 6%, 9% | 6 oz, 12 oz | ||
Pads | 3%, 6%, 9% | 1 g (30 or 60/box) | ||
Foaming cloths | 3%, 6%, 9% | 3.2 g (30 or 60/box) | ||
Zoderm | Cleanser | 4.5%. 6.5%, 8.5% | 400 mL | |
Pads | 4.5%. 6.5%, 8.5% | 6 mL (30/box) | ||
Hydrating wash | 5.75% | 400 mL | ||
Generic | Gel | 5%, 10% | 45 g, 60 g, 90 g | |
Wash | 2.5%, 5%, 10% | 142 g, 227 g | ||
Erythromycin | Generic | Gel | 2% | 30 g, 60 g |
Ointment | 2% | 25 g | ||
Solution | 2% | 60 mL | ||
Pledget | 2% | (60/box) | ||
Clindamycin | Cleocin T | Gel | 1% | 30 g, 60 g |
Lotion | 1% | 60 mL | ||
Solution | 1% | 30 mL, 60 mL | ||
Pledget | 1% | (60/box) | ||
Evoclin | Foam | 1% | 50 g, 100 g | |
Clindagel | Gel | 1% | 40 mL, 75 mL | |
ClindaMax | Gel | 1% | 30 g, 60 g | |
Lotion | 1% | 60 mL | ||
Clindets | Pledget | 1% | 60 s | |
Generic | Gel | 1% | 30 g, 60 g | |
Lotion | 1% | 30 g, 60 g | ||
Solution | 1% | 30 g | ||
Dapsone | Aczone | Gel | 5% | 30 g |
Benzoyl peroxide/erythromycin | Benzamycin Benzamycin Gel Pak | Gel | 5%/3% | 46.6 g, 60/box |
Generic | Gel | 5%/3% | 23.2 g, 46.6 g | |
Benzoyl peroxide/clindamycin | Benzaclin | Gel | 5%/1% | 25 g, 50 g 50-g pump |
Duac | Gel | 5%/1% | 45 g | |
Acanya | Gel | 2.5%/1.2% | 50 g | |
Generic | Gel | 5%/1% | 50 g | |
Benzoyl peroxide/hydrocortisone | Vanoxide HC | Lotion | 5%–0.5% | 25 mL |
Miscellaneous | ||||
Sodium sulfacetamide | Klaron | Lotion | 10% | 4 oz |
Sodium sulfacetamide/sulfur | Sulfacet-R | Lotion | 10–5% | 25 g |
Rosula | Gel | 10%–5% in 10% urea | 45 mL | |
Cleanser | 10%–5% in 10% urea | 355 mL | ||
Azeleic acid | Azelex | Cream | 20% | 30 g, 50 g |
Products containing sulfur, sodium sulfacetamide, and resorcinol, once favored treatments for acne, are still found in several over-the-counter and prescription niche formulations. Sulfonamides are thought to have antibacterial properties through their inhibition of para-aminobenzoic acid (PABA), an essential substance for P. acnes growth.52 Sulfur also inhibits the formation of free fatty acids and has presumptive keratolytic properties. It is often combined with sodium sulfacetamide to enhance its cosmetic tolerability due to sulfur’s distinctive odor. Resorcinol is also indicated for use in acne for its antimicrobial properties. It is generally found in 2% concentration in combination with 5% sulfur.
Salicylic acid is a ubiquitous ingredient found in over-the-counter acne preparations in concentrations ranging from 0.5% to 2%. This lipid soluble β-hydroxy acid has comedolytic properties, though somewhat weaker than those of a retinoid. Salicylic acid also causes exfoliation of the stratum corneum though decreased cohesion of the keratinocytes. Mild irritant reactions may result.
Azelaic acid is available by prescription in a 20% cream or 15% gel. This dicarboxcylic acid has both antimicrobial and comedolytic properties.53 It is also a competitive inhibitor of tyrosinase and thus may decrease postinflammatory hyperpigmentation.54 It is generally well tolerated, though transient burning can occur, and is safe in pregnancy.
Benzoyl peroxide preparations are among the most common topical medications prescribed by dermatologists and are also readily available over-the-counter. Benzoyl peroxide is a powerful antimicrobial agent through decreasing both the bacterial population and the hydrolysis of triglycerides. Benzoyl peroxide preparations are available in creams, lotion, gels, washes, and pledgets. Products that are left on the skin, such as a gel, are generally considered more effective. Benzoyl peroxide can produce significant dryness and irritation. Allergic contact dermatitis has been uncommonly reported. Of significance, bacteria are unable to develop resistance to benzoyl peroxide, making it the ideal agent for combination therapy.55
(See Chapter 218).
Erythromycin and clindamycin are the most commonly used topical antibiotics for the treatment of acne. These two agents have also been used in combination preparations with benzoyl peroxide. Increased levels of P. acnes resistance have been reported in patients who are being treated with antibiotics. However, the development of resistance is less likely in patients who are treated with a combination of benzoyl peroxide/erythromycin or clindamycin.56 Therefore, the combination of these two products is preferable over monotherapy with topical antibiotics. Topical dapsone is the most recently approved topical antibiotic for acne. With twice daily application topical dapsone has shown better efficacy in controlling inflammatory lesions (58%) versus noninflammatory lesions (19%).57,58 Unlike oral dapsone, topical dapsone is safe for use even in patients with a G6PD deficiency.59 It is generally well tolerated but should not be applied concomitantly with benzoyl peroxide or it may impart an orange color on the skin.60
(See Chapter 217).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree