Fig. 43.1
(a) Skin island designed over anterior abdominal wall muscles. (b) TAW flap harvested on SIEA bilaterally. (c) SIEAs on posterior view of TAW flap. (d) Schematic presentation of TAW flap. (e) The silicon sheet lying over anterior abdominal muscle from the xsphoid process to pubic tubercle. TAW total abdominal wall, SEA superficial epigastric artery, SEV superficial epigastric vein, XP xsifoid process, PT pubic tubercle
In the second group B, diameters of total abdominal wall skin were compared with previous CTA models, which included skin component. For this purpose, hemi face, full face, groin, and limb transplant models were performed in Lewis (RT11) rats (n = 2). Entire skin component was harvested from limb transplant model. Next total cross-section areas of skin components in all flaps were measured using millimetric graph paper and digital photography were taken.
Experimental Group
Two subgroups were prepared as isograft and allograft transplant. Isograft transplants were performed between Lewis (RT11) rats (n = 8). Total abdominal wall transplants were elevated as described above and femoral vessels were transected under the inguinal ligament. For recipient vessels, keeping bilateral femoral arteries and veins as long as possible, they were transected before the bifurcation at popliteal region. Total abdominal wall skin (approximately 12 × 8 cm) was resected from recipient rat to make defect in proportion to donor flap size. Femoral arteries and veins of donor and recipient were anastomosed using end-to-end technique with 10/0 nylon. TAW flap was sutured into created defect with absorbable suture material (4/0 Vicryl, Ethicon, a Johnson & Johnson company, Somerville, NJ). In allograft transplant group (n = 8), TAW flaps were transplanted from LBN (RT1+n) donor to Lewis (RT11) recipients. To avoid seroma complication that we observed in the first two experiments (Fig. 43.2a), a few suture were placed with absorbable suture material (4/0 Vicryl, Ethicon) between flap and anterior abdominal wall to decrease the dead space and provide close contact (Fig. 43.2b). For immunosuppression protocol, Cyclosporine A monotherapy was used with tapered dosage (from 16 to 2 mg/kg/day).
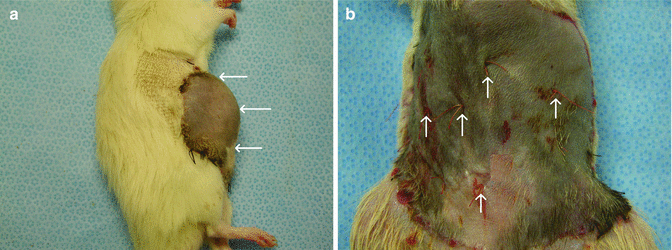

Fig. 43.2
(a) Expanded flap with seroma (white arrows) at postransplant second week. (b) Sutures (white arrows) for fixing the flap with abdominal muscle
Assessment of Flap Viability
Direct Observation
Viability of the skin island was followed by daily observation of both iso and allograft transplants by the same surgeon. The survival of the TAW skin transplant was evaluated during postoperative follow-up period by gross inspection of the flap color, edema, erythema, hair loss, necrosis compared with normal skin.
Microangiography
Microangiography was performed to delineate the vascularization of the total abdominal wall flap in two animals in isograft transplant groups on posttransplant day 15. After cannulating the common carotid artery, 50 mL of the mixture (at 70 °C) that was made of 50 g of silver oxide, 5 g of gelatin, and 100 mL of the isotonic was injected with a syringe. The flaps were stored at 4 °C for 4 h. Then flaps underwent radiography with soft x-ray machine, using Microvision-C mammography film.
Indian Ink Injection Studies
In two isograft transplants, viability of flaps was evaluated by the presence of dye in the vessels after India ink injection. Following cannulation of the common carotid
Histology
On posttransplant day 100, two animals in allograft group were euthanized via intracardiac lidocaine injection under general anesthesia. Flaps were harvested and placed in 10 % buffered formalin. The skin sections were stained with H&E for evaluation.
Immunologic Evaluation
Flow cytometry was used to evaluate the presence of donor-specific chimerism for donor MHC class I (RT1n) antigen in the peripheral blood of Lewis abdominal wall transplant recipients during observation time at day 7, 21, 63, and 100 posttransplant. Donor chimerism was assessed with combinations of conjugated mouse antirat mAb RT1n-FITC (for MHC class I of donor cells RT1n, clone MCA156; Serotec, UK) with mAb specific for T-cells: CD4−PE (clone OX-35), CD8a−PE (clone OX-8), B-cell CD45RA−PE (clone OX-33) and monocytes, granulocytes CD11b/c −PE(clone OX-42) (BD Bioscience Pharmingen). After incubation, samples were lysed and fixed with 1 % PFA solution. Negative control panels were tested and included isotype-matched antibodies (IgG−FITC/IgG−PE) and phosphate-buffered saline samples. Analysis was performed on 1 × 104 cells using FACS SCAN (BD Bioscience Pharmingen) and Cell Quest software. All results of flow cytometry at posttransplant period were compared with data of hemiface and groin flaps. These data were taken from our previously study and this studies were used same immunosuppressive protocol [8, 9]. Blood chimerism level of limb allotransplant were not added this study. Because these transplants include vascularized bone marrow and this component may cause different immunologic result compare with CTA transplant models without vascularized bone marrow.
Results
Anatomic Studies
At postoperative 21 day, all skin islands were viable for total abdominal wall flaps which were based bilaterally on SEV pedicles (Fig. 43.3a). Half of skin islands were necrotic in the remaining flaps, which had only one pedicle (Fig. 43.3b). In dye studies, total and half of skin island of TAW flap which had two and one SEVs, respectively, were stained with India ink (Fig. 43.4a, b) These results demonstrated that of TAW flaps bilateral SEVs for total flap viability. In measurement of skin islands of CTA different models, skin diameters were found from the largest to smallest diameter as 27.92 cm2, 12 cm2, 6.72 cm2, 5.84 cm2, and 5.20 cm2 for TAW, total face, limb transplant, hemiface, groin, respectively (Table 43.1) (Fig. 43.5).
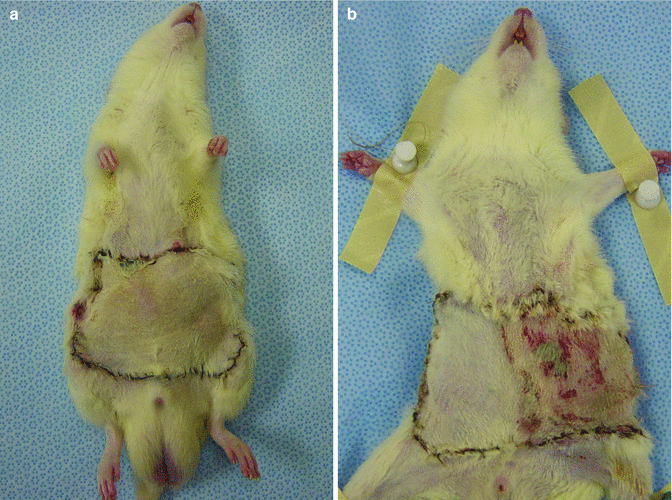
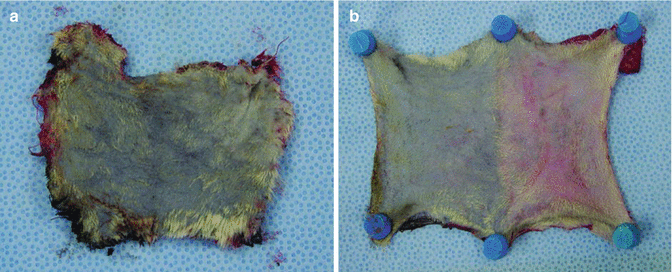
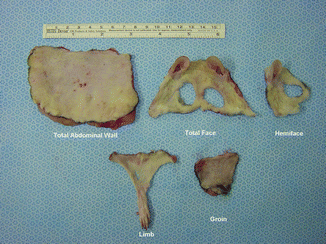

Fig. 43.3
(a) Total viable skin on intact bilaterally pedicle. (b) Necrotic skin area on the transected pedicle side

Fig. 43.4
View of skin island of abdominal wall. Stained whole (a) and half of skin island (b) in TAW which had two and one SIE vessels respectively
Table 43.1
Comparing of skin surface area of CTA transplants
CTA transplant model | Surface area (cm2) |
|---|---|
TAW | 27.92 |
Total face | 12 |
Limb transplant | 6.72 |
Hemiface | 5.84 |
Groin | 5.20 |

Fig. 43.5
Comparing skin islands of CTA transplants
Experimental Group
Direct Observation
On postoperative follow-up period (at posttransplant 100 day) of skin island of all flaps in isograft and allograft transplant groups was completely viable (Fig. 43.6a, b).
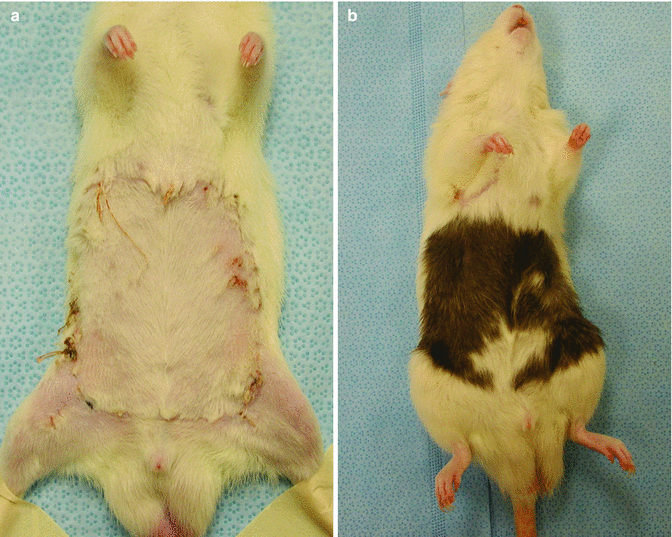

Fig. 43.6
Viable skin islands of isograft (a) and allograft (b) transplants at late follow up period
Microangiography
Microangiography revealed the vascular supply in flap as provided by the pedicles (Fig. 43.7). Indian Ink injection Studies Dye study with India ink demonstrated ink uptake by the vessels in skin, confirming perfusion via bilateral pedicle (Fig. 43.8).
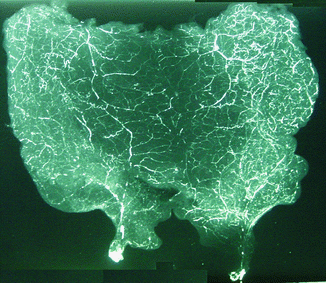
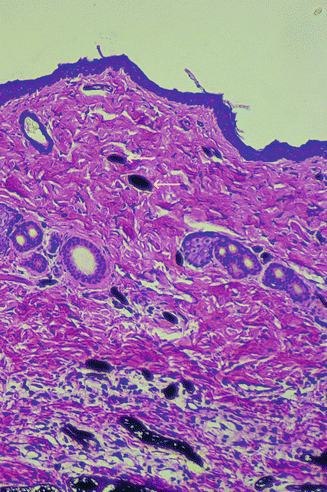

Fig. 43.7
Microangiographic analysis reveals courses of pedicles

Fig. 43.8
Histological examination of flap skin indicates perfusion by presence of ink in vessels (arrows)









