Histology.
Early histopathological changes in HS are follicular hyperkeratosis of a dilated infundibulum with plugging. A subsequent bacterial superinfection and rupture of the follicle is then seen, resulting in inflammation of the connective tissue. A mixed perifollicular lymphohistiocytic infiltrate with plasma cells, mononuclear cells and neutrophilic granulocytes is seen, with an acanthotic and anastomosing interfollicular epithelium [3,7].
Aetiology.
As evidenced by the different nomenclature used to identify this disorder, the aetiology of hidradenitis suppurativa/acne inversa is unclear. While affected areas reflect the anatomical distribution of apocrine sweat glands, histology suggests that apocrine glands are not the primary source of pathology. Hidradentis suppurativa has rather been identified as a follicular hyperkeratosis with plugging and dilation of the hair follicle, precipitating inflammation, abscess and sinus tract formation. Involvement of the apocrine glands is secondary, resulting in deeper granulomatous inflammation.
The aetiology of follicular plugging is unclear. Proposed mechanisms such as hypersecretion of sebum, proliferation of Propionibacterium acnes in the setting of an alteration of innate immunity, and inflammatory reactions, mirror those implicated in the pathogenesis of acne vulgaris [8].
Hidradentis suppurativa has a clear genetic component with multiple familial cases being reported, implying an autosomal dominant inheritance pattern [9]. A recent report from two case–control studies in France also identified two risk factors significantly associated with HS: current smoking and obesity [2].
Differential Diagnosis.
At the initial stages, the differential diagnosis should include infectious lesions such as furuncles and carbuncles, deep fungal infections, actinomycosis and sportotrichosis. Lesions in the anogenital region are reminiscent of lymphogranuloma venereum and granuloma inguinale. Sweat gland abscesses, vegetating pyoderma, cutaneous tuberculosis, irritated sebaceous gland retention cysts and cutaneous fistulas in Crohn disease should also be ruled out [3,9].
Management.
First-line treatment consists of lifestyle modification including appropriate hygiene, loose-fitted clothing, smoking cessation and weight loss when relevant [10]. Drainage incisions parallel to skin folds will aid in the resolution of the acute inflammation secondary to infection. Short-course antibiotics should be used for cases complicated by cellulitis. Topical clindamycin and systemic tetracycline-class antibiotics have been used with success. Adjuctive therapies include the use of retinoids, intralesional injection of triamcinolone acetonide suspension, antiandrogens and infliximab [11]. Excision to fascia can be effective in chronic or refractory disease [4].
Prognosis.
Healing of areas affected by HS with scarring can lead to contractures and significant limitation in mobility. There is a recurrence rate of 2.5% after wide surgical excision. Ninety per cent of patients with HS will continue to have symptomatic disease years after initial presentation. There is a 3% prevalence of squamous cell carcinoma in patients with long-standing perianal HS [4].
References
1 Lam J, Krakowski AC, Friedlander SF. Hidradenitis suppurativa (acne inversa): management of a recalcitrant disease. Pediatr Dermatol 2007;24:465–73.
2 Revuz JE, Canoui-Poitrine F, Wolkenstein P, et al. Prevalence and factors associated with hidradenitis suppurativa: results from two case–control studies. J Am Acad Dermatol 2008;59:596–601.
3 Meixner D, Schneider S, Krause M, Sterry W. Acne inversa. J Dtsch Dermatol Ges 2008;6:189–96.
4 Golladay ES. Outpatient adolescent surgical problems. Adolesc Med Clin 2004;15:503–20.
5 Mengesha YM, Holcombe TC, Hansen RC. Prepubertal hidradenitis suppurativa: two case reports and review of the literature. Pediatr Dermatol 1999;16:292–6.
6 Palmer RA, Keefe M. Early-onset hidradenitis suppurativa. Clin Exp Dermatol 2001;26:501–3.
7 Kurokawa I, Nishijima S, Kusumoto K et al. Immunohistochemical study of cytokeratins in hidradenitis suppurativa (acne inversa). J Int Med Res 2002;30:131–6.
8 Kurzen H, Kurokawa I, Jemec GB et al. What causes hidradenitis suppurativa? Exp Dermatol 2008;17:455–6; discussion 457–72.
9 Prasad PV, Kaviarasan PK, Joseph JM, Madhuri S, Viswanathan P. Familial acne inversa with acne conglobata in three generations. Indian J Dermatol Venereol Leprol 2008;74:283–5.
10 Slade DE, Powell BW, Mortimer PS. Hidradenitis suppurativa: pathogenesis and management. Br J Plast Surg 2003;56:451–61.
11 Antonucci A, Negosanti M, Negosanti L, Iozzo I, Varotti C. Acne inversa treated with infliximab: different outcomes in 2 patients. Acta Derm Venereol 2008;88:274–5.
Fox–Fordyce Disease
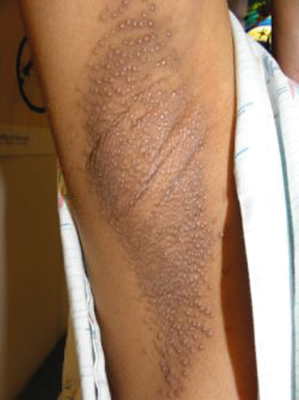
Fox–Fordyce disease (FFD) or apocrine miliaria is a rare and chronic disorder characterized by inflammation of the apocrine sweat glands causing pruritic follicular papules confined to apocrine gland-bearing areas.
Epidemiology.
Fox–Fordyce is an extremely rare disease, most common in women between 13 and 35 years of age [1]. Cases in prepubescent girls have been reported [1,2], highlighting the fact that hormonal factors may not be operative in all cases. Pregnancy has been found to be protective.
Presentation.
Fox–Fordyce disease is characterized by pruritic small 1–3 mm conical flesh-coloured to slightly yellow follicular papules on a slightly erythmatous base in the apocrine gland-bearing region (axillae, groins, pubic region, perineum, labia morjora, areola mammae and umbilicus) (Fig. 94.2). Most commonly affected regions are the axillae and areolae [3]. Hairs are usually sparse in affected areas, most likely because of rubbing and scratching. Lesions are often refractory to treatment and spontaneous resolution is not expected.
Aetiology.
Fox–Fordyce disease is caused by obstruction of the apocine duct at the entrance into the follicular wall [4]. Subsequent apocrine sweat retention leads to rupture of the duct causing a secondary inflammatory reaction within the dermis. Alternative theories hypothesize an inflammatory process induces a reactive hyperkeratosis. Proposed influences include emotional factors, hormonal influences and chemical changes in sweat composition.
Diagnosis.
Fox–Fordyce disease is diagnosed based on its characteristic clinical features. Histopathological features of FFD, while well described, are less consistently observed.
Pathology.
The histopathological findings of FFD have been traditionally described as infundibular plugging, acanthosis, parakeratosis, spongiosis, with a non-specific inflammatory infiltrate [3,4]. The ‘sweat retention vesicle’ described by Shelley has been proposed as a singular diagnostic feature [5]. Additional findings of scattered infundibular dyskeratotic cells, vacuolar alteration at the junction between infundibular epithelium and its adventitia, and cornoid lamella-like parakeratosis within the infundibular plug have been reported by Boer. Recently, perifollicular xanthomatosis have been proposed as a representative hallmark of FFD. Perifollicular mucin, adventitial fibrosis and increased mast cell density may also be used as clues to aid in diagnosis [3].
Differential Diagnosis.
While the clinical features of FFD are characteristic and unique, folliculitis, lichen nitidus, miliara rubra, lichen simplex, lichen planus and syringoma should be considered [4,7].
Treatment.
Treatment of FFD has been uniformly unsatisfactory. Goals are aimed at improving pruritus and decreasing the size and number of the lesions. Reported treatments include oral antihistamines, topical, systemic and intralesional steroids, keratolytic agents (salicylic acid, propylene glycol), ultraviolet light treatment, oral contraceptives, topical clindamycin [8], topical tretinoin, isotretinoin, topical 5% benzoyl peroxide in combination with loratidine [4]. Pimecrolimus has also been reported to have benefit [6]. Surgical treatment, electrocoagulation, laser therapy, dermabrasion [8] and liposuction-assisted curettage [9] have all been tried with variable results.
References
1 Ranalletta M, Rositto A, Drut R. Fox–Fordyce disease in two prepubertal girls: histopathologic demonstration of eccrine sweat gland involvement. Pediatr Dermatol 1996;13:294–7.
2 Sandhu K, Gupta S, Kanwar AJ. Fox–Fordyce disease in a prepubertal girl. Pediatr Dermatol 2005;22:89–90.
3 Bormate AB Jr, Leboit PE, McCalmont TH. Perifollicular xanthomatosis as the hallmark of axillary Fox–Fordyce disease: an evaluation of histopathologic features of 7 cases. Arch Dermatol 2008;144:1020–4.
4 Ozcan A, Senol M, Aydin NE, Karaca S, Sener S. Fox–Fordyce disease. J Eur Acad Dermatol Venereol 2003;17:244–5.
5 Shelley WB, Levy EJ. Apocrine sweat retention in man. II. Fox–Fordyce disease (apocrine miliaria). AMA Arch Derm 1956;73:38–49.
6 Pock L, Svrcková M, Machácková R, Hercogová J. Pimecrolimus is effective in Fox–Fordyce disease. Int J Dermatol 2006;45:1134–5.
7 Kineston DP, Martin KO. Pruritic axillary papules: Fox–Fordyce disease. Am Fam Physician 2008;77:1735–6.
8 Miller ML, Harford RR, Yeager JK. Fox–Fordyce disease treated with topical clindamycin solution. Arch Dermatol 1995;131:1112–3.
9 Chae KM, Marschall MA, Marschall SF. Axillary Fox–Fordyce disease treated with liposuction-assisted curettage. Arch Dermatol 2002;138:452–4.
Tumours
Follicular Differentiation
Trichofolliculoma
Trichofolliculoma is a rare benign adnexal hamartoma of hair follicle origin with differentiation toward hair production [1,2].
Epidemiology.
While trichofolliculoma is generally perceived as a disorder of the adult population, a single case of congenital trichofolliculoma has been reported in the literature [3]. A review of 29 cases reported a mean age at time of removal of 44 years [2].
Presentation.
Trichofolliculoma typically present as a single 2–5 mm skin-coloured nodule on the head or neck, especially in the area of the nose, cheek, scalp and eyeline [1,4]. Occasionally, wool-like wisps of immature hairs emerge from a central orifice [1].
Aetiology.
Trichofolliculoma is considered an intermediate differentiation between a hair follicle naevus, simple hyperplasia of the hair follicle and trichoepithelioma, which usually lacks mature hair follicles [4]. Studies examining cytokeratin expression in trichofolliculoma reveal differentiation towards the hair bulge and the outer root sheath in the isthmus [5]. After examining multiple lesions, Hartschuh and Schulz [2] developed the concept of trichofolliculoma as a lesion that undergoes great morphological changes that correspond to the normal hair follicle.
Diagnosis.
Diagnosis is based upon histopathology.
Pathology.
Histology reveals one or several keratin-filled cysts or sinuses in the dermis (primary follicle), which is lined by squamous epithelium. Many small well-differentiated hair follicles (secondary follicles) radiate from the wall of these sinuses. Trichofolliculoma generally differentiates in the direction of the outer root sheath [5]. Merkel cell hyperplasia can be a diagnostic aid helping to differentiate later stage trichofolliculomas from fibrofolliculomas and fibrous papules [2].
Differential Diagnosis.
Differential diagnosis of this disorder includes hair follicle naevus, trichofolliculoma, fibrous papule and fibrofolliculomas.
Treatment.
Surgical excision is standard of care.
Prognosis.
Trichofolliculoma has a generally benign course [4]. Extraction of associated hairs may promote inflammatory reactions [1].
References
1 Carreras B Jr, Lopez-Marin I Jr, Mellado VG, Gutierrez MT. Trichofolliculoma of the eyelid. Br J Ophthalmol 1981;65:214–15.
2 Hartschuh W, Schulz T. Immunohistochemical investigation of the different developmental stages of trichofolliculoma with special reference to the Merkel cell. Am J Dermatopathol 1999;21:8–15.
3 Ishii N, Kawaguchi H, Takahashi K, Nakajima H. A case of congenital trichofolliculoma. J Dermatol 1992;19:195–6.
4 Mizutani H, Senga K, Ueda M. Trichofolliculoma of the upper lip: report of a case. Int J Oral Maxillofac Surg 1999;28:135–6.
5 Kurokawa I, Kusumoto K, Sensaki H et al. Trichofolliculoma: case report with immunohistochemical study of cytokeratins. Br J Dermatol 2003;148:597–8.
Desmoplastic Trichoepithelioma
Trichoepitheliomas are rare benign adnexal tumours of follicular differentiation. Three distinct subtypes of trichoepitheliomas have been described: solitary, multiple and desmoplastic [1]. The solitary variant, usually seen only in the adult population, has been described as a skin-coloured 5–8 mm diameter perinasal papule. Multiple trichoepitheliomas, morphologically identical to the solitary variants, are seen in various genetic disorders including cylindromatosis and Rombo syndrome, and usually appear during adolescence. The third variant, desmoplastic trichoepithelioma (DTE), first described by Brownstein and Shapiro in 1977 [2], present as firm annular asymptomatic 3–8 mm diameter plaques. This section will address solitary DTE.
Epidemiology.
Desmoplastic trichoepithelioma has an incidency of 1 in 5000 skin biopsies in adults. Desmoplastic trichoepithelioma most commonly occur in middle-aged women [3]; patients range in age at diagnosis from 8 to 70 years with a median age of 46, with 85% of cases seen in females. Familial DTE is extremely rare [4]. Cases of DTE have been reported in infants with improvement of physical appearance of the lesions with the ageing of the child [5,6].
Presentation.
Desmoplastic trichoepithelioma are firm symmetric oval asymptomatic white to yellow papules or plaques. Lesions are indurated with depressed non-ulcerated centres and raised or rolled borders. They are predominantly found on the face (cheeks, chin and forehead) but can also been seen on the scalp, neck and upper trunk areas [3]. Lesions enlarge slowly over many years and rarely are observed larger than 2 cm. Desmoplastic trichoepithelioma is assocated with intradermal naevus at a frequency of 10% [4]. Milia-like components may also surround the lesions [5].
Aetiology.
Histological examination of DTE has provided clues as to the aetiology of this disorder. The presence of Merkel cells indicates a bulge of isthmus-derived origin. The cells in DTE are suggested to be in close association with the basal cells in the outer root sheath which can subsequently differentiate into parts of the folliculosebaceous unit [4]. Reports of familial DTE imply an unknown genetic influence.
Diagnosis.
Diagnosis is based on clinical and histological features. Dermoscopy may aid in the diagnosis, revealing lesions with a distinguishing pearl-white to ivory-white colour and prominent, large arborizing vessels, characteristics that are absent in basal cell carcinoma (BCC) [1].
Pathology.
Histologically, DTE share some features with BCC. The following triad describe DTE: narrow strands of trichoblast tumour cells, keratinaceous cysts and desmoplastic stroma [2]. Other characteristics include horn cysts, epidermal hyperplasia, foreign body keratin granulomas and calcification. Sebaceous and apocrine differentiation is occasionally seen along with the usual follicular differentiation. Aggregations are rimmed by thin bundles of collagen that are separated from the surrounding dermis by a cleft [7]. Absence of androgen receptor expression in DTE helps to differentiate this lesion from BCC [8].
Differential Diagnosis.
The clinical and histological features of DTE are most similar to those of morpheaform BCC. One must also consider sebaceous hyperplasia, conventional trichoepithelioma, granuoma annulare, scar tissue, scleroderma and cutaneous sarcoidosis [3,4].
Treatment.
Desmoplastic trichoepitheliomas do not require treatment. However, many patients undergo treatment for cosmetic reasons. Standard treatment is surgical excision [4]. Curretage and electrodessication has also been used with no recurrence reported [5]. Dermabrasion with laser surgery has been reported to have success [3].
References
1 Ardigo M, Zieff J, Scope A et al. Dermoscopic and reflectance confocal microscope findings of trichoepithelioma. Dermatology 2007;215:354–8.
2 Brownstein MH, Shapiro L. Desmoplastic trichoepithelioma. Cancer 1977;40:2979–86.
3 Koay JL, Ledbetter LS, Page RN, Hsu S. Asymptomatic annular plaque of the chin: desmoplastic trichoepithelioma. Arch Dermatol 2002;138:1091–6.
4 Wang SH, Tsai RY, Chi CC. Familial desmoplastic trichoepithelioma. Int J Dermatol 2006;45:756–8.
5 Carter JJ, Kaur MR, Hargitai B et al. Congenital desmoplastic trichoepithelioma. Clin Exp Dermatol 2007;32:522–4.
6 Chuo CB, Slator R, Brown RM, Anderson KD. Management of desmoplastic trichoepithelioma in an infant. J Plast Reconstr Aesthet Surg 2008;61:1241–4.
7 Costache M, Bresch M, Boer A. Desmoplastic trichoepithelioma versus morphoeic basal cell carcinoma: a critical reappraisal of histomorphological and immunohistochemical criteria for differentiation. Histopathology 2008;52:865–76.
8 Izikson L, Bhan A, Zembowicz A. Androgen receptor expression helps to differentiate basal cell carcinoma from benign trichoblastic tumors. Am J Dermatopathol 2005;27:91–5.
Hair Follicle Naevus
Hair follicle naevi are rare benign often congenital hamartomas of follicular differentiation composed of multiple vellus hairs.
Epidemiology.
Hair follicle naevi are often congenital, presenting most commonly in infancy as a single nondescript 3–7 mm skin-coloured papule [1]. While generally considered a congenital lesion, cases of acquired lesions have been reported [2,3].
Presentation.
Hair follicle naevi most commonly present as a nodule on the face within the distribution of the first branchial arch [2]. Although usually a solitary lesion, reports of multiple lesions have been described [4], including those that follow the lines of Blaschko [5]. Association with leptomeningeal angiomatosis [6] and other epidermal lesions [4,5] has also been reported.
Aetiology.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree