Anticonvulsant agents, NSAIDs, allopurinol, corticosteroids, moxifloxacin
Anticonvulsant agents, allopurinol, corticosteroids, carbamazepine, modafinil, NSAIDs (especially piroxicam), lamotrigine, phenytoin, minocycline
Pathogenesis.
Erythema multiforme and some cases of SJS, especially those with recurrent episodes, have been strongly associated with activity of the herpes simplex virus (HSV) and Mycoplasma pneumoniae, or drug-specific T-helper (Th) 1 lymphocytes. Gelatinase activity has also been demonstrated in EM lesions, with tissue levels and patterns similar to those in SJS/TEN [22,23].
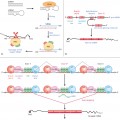
In severe drug reactions such as SJS/TEN, apoptosis of keratinocytes is a common histopathological feature, and this keratinocyte death is now known to be mediated by fatty acid synthetase ligand (FasL). A recent report showed that soluble FasL (sFasL) is a useful tool for differentiating viral exanthems from drug eruptions as it was found to be repeatedly negative in viral exanthems but present in SJS/TEN [24]. A Japanese study has found FasL gene polymorphisms associated with SJS/TEN cases [25]. The main pathomechanism is thought to be perforin-secreting CD8+ T-lymphocyte coupled with FasL CD40L+ Th1/Th2 lymphocyte activity [22,26]. The latest concept is that granulysin expressed by cytotoxic T-lymphocytes and natural killer cells may be the key mediator for disseminated keratinocyte death as it was found in blister fluid of SJS/TEN patients, at 2–4 orders of magnitude higher than perforin, granzyme B or soluble FasL [27].
References
1 Von Hebra F. Atlas der Hautkrankheiten. Vienna: Kaiserliche Akademie der Wissenchafte, 1866.
2 Roujeau JC. What is going on in erythema multiforme? Dermatology 1994;188:249–50.
3 Stevens AM, Johnson FC. A new eruptive fever associated with stomatitis and ophthalmia. Am J Dis Child 1922;24:526–33.
4 Lyell A. Toxic epidermal necrolysis: an eruption resembling scalding of the skin. Br J Dermatol 1956;68:355–61.
5 Lyell A. Requiem for toxic epidermal necrolysis. Br J Dermatol 1990;122:837–46.
6 Roujeau JC. Stevens–Johnson syndrome and toxic epidermal necrolysis are severity variants of the same disease which differs from erythema multiforme. J Dermatol 1997;24:726–9.
7 Auquier-Dunant A, Mockenhaupt M, Naldi L et al. Correlations between clinical patterns and causes of erythema multiforme majus, Stevens–Johnson syndrome, and toxic epidermal necrolysis: results of an international prospective study. Arch Dermatol 2002;138:1019–24.
8 Bastuji-Garin S, Rzany B, Stern SR et al. Clinical classification of cases of toxic epidermal necrolysis, Stevens–Johnson syndrome, and erythema multiforme. Arch Dermatol 1993;129:92–6.
9 Rzany B, Hering O, Mockenhaupt M et al. Histopathological and epidemiological characteristics of patients with erythema exudativum multiforme major, Stevens–Johnson syndrome and toxic epidermal necrolysis. Br J Dermatol 1996;135:6–11.
10 Wolf R, Wolf D, Davidovici B. In the pursuit of classifying severe cutaneous adverse reactions. Clin Dermatol 2007;25:348–9.
11 Léauté-Labrèze C, Lamireau T, Chawki D et al. Diagnosis, classification, and management of erythema multiforme and Stevens–Johnson syndrome. Arch Dis Child 2000;83:347–52.
12 Huff JC, Weston WL, Tonnesen MG et al. Erythema multiforme: a critical review of characteristics, diagnostic criteria, and causes. J Am Acad Dermatol 1983;8:763–75.
13 Letko E, Papaliodis DN, Papaliodis GN et al. Stevens–Johnson syndrome and toxic epidermal necrolysis: a review of literature. Ann Allergy Asthma Immunol 2005;94:419–36.
14 Borchers AT, Lee JL, Naguwa SM et al. Stevens–Johnson syndrome and toxic epidermal necrolysis. Autoimmun Rev 2008;7:598–605.
15 Levi N, Bastuji-Garin S, Mockenhaupt M et al. Medications as risk factors of Stevens–Johnson syndrome and toxic epidermal necrolysis in children: a pooled analysis. Pediatrics 2009;123:e297–e304.
16 Lonjou C, Thomas L, Borot N et al. A marker for Stevens–Johnson syndrome … : ethnicity matters. Pharmacogenomics J 2006;6:265–8.
17 Ferrell PB Jr, McLeod HL. Carbamazepine, HLA–B*1502 and risk of Stevens–Johnson syndrome and toxic epidermal necrolysis: US FDA recommendations. Pharmacogenomics 2008;9:1543–6.
18 Halevy S, Ghislain PD, Mockenhaupt M et al. Allopurinol is the most common cause of Stevens–Johnson syndrome and toxic epidermal necrolysis in Europe and Israel. J Am Acad Dermatol 2008;58:25–32.
19 Lonjou C, Borot N, Sekula P et al. A European study of HLA-B in Stevens–Johnson syndrome and toxic epidermal necrolysis related to five high-risk drugs. Pharmacogenet Genom 2008;18:99–107.
20 Dore J, Salisbury RE. Morbidity and mortality of mucocutaneous diseases in the pediatric population at a tertiary care center. J Burn Care Res 2007;28:865–70.
21 Mirakian R, Ewan PW, Durham SR et al. BSACI guidelines for the management of drug allergy. Clin Exp Dermatol 2009;39:43–61.
22 Caproni M, Torchia D, Volpi W et al. Expression of matrix metalloproteinases 2, 9, 11 in erythema multiforme: immunohistochemical comparison with Stevens–Johnson syndrome/toxic epidermal necrolysis. Br J Dermatol 2008;158:1163–6.
23 Quaglino P, Caproni M, Antiga E et al. Serum levels of the Th1 promoter IL-12 and the Th2 chemokine TARC are elevated in erythema multiforme and Stevens–Johnson Syndrome/Toxic Epidermal Necrolysis and correlate with soluble Fas Ligand expression. Dermatology 2007;214:296–304.
24 Stur K, Karlhofer FM, Stingl G. Soluble Fas ligand: a discriminating feature between drug-induced skin eruptions and viral exanthemas. J Invest Dermatol 2007;127:802–7.
25 Ueta M, Sotozono C, Inatomi T et al. Association of Fas ligand gene polymorphism with Stevens Johnson syndrome. Br J Ophthalmol 2008;92:989–91.
26 Posadas SJ, Padial A, Torres MJ et al. Delayed reactions to drugs show levels of perforin, granzyme B, and Fas-L to be related to disease severity. J Allergy Clin Immunol 2002;109:155–61.
27 Chung WH, Hung SI, Yang JY et al. Granulysin is a key mediator for disseminated keratinocyte death in Stevens–Johnson syndrome and toxic epidermal necrolysis. Nat Med 2008;14:1343–50.
Erythema Multiforme
Erythema multiforme (EM), as originally described by von Hebra in 1866, is a self-limited condition characterized by the abrupt onset of red papules which evolve into target lesions, with a tendency to recur. EM is classified into ‘EM minor’ and ‘EM major’ in an attempt to separate the classic, mild disease described by von Hebra (EM minor), which is most often associated with herpes simplex virus (HSV) infection in almost 50% of cases, and the more severe form with mucosal involvement usually attributed to Mycoplasma pneumoniae infections and drugs [1,2]. To a lesser degree, there are a multitude of other infections reported in association with EM, including those caused by other viruses (i.e. Epstein–Barr virus (EBV), vaccinia and other herpes viruses), and certain bacteria, mycobacteria and fungi [1,3].
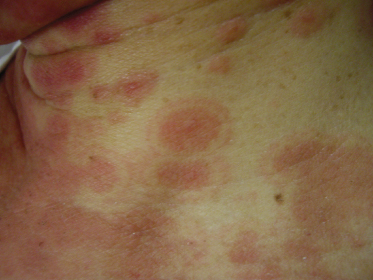
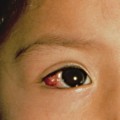
Patients with EM may experience burning or itching at the site of the eruption, which usually appears symmetrically on the distal extremities, gradually progressing proximally. The involvement of the dorsal hands, as well as the extensor surfaces of the extremities, is common, as is involvement of the palms and soles. The individual lesions may start off as erythematous macules that later evolve into papules, plaques and finally target lesions, which are only seen several days after the onset [2]. There are usually lesions of varying morphology co-existing, hence the term erythema ‘multiforme’. The target lesions initially described consisted of two distinct zones: an inner zone of acute epidermal injury with necrosis or blisters and an outer zone of erythema [4]. Recent descriptions of target lesions have mentioned three distinct zones: a dusky area of central necrosis, a middle zone of pale oedema and an outer zone of erythema [2,5]. Figure 78.1 shows typical target lesions in a patient with EM.
Differential diagnosis includes drug eruption, polymorphous light eruption, urticaria, urticarial vasculitis and other viral exanthems. As EM is generally self-limiting, management rarely requires hospital admission. In a 10-year review of EM, SJS and TEN in children, 300,000 records were reviewed and only 30 cases of EM required admission and no mortality was reported [3]. In a Taiwanese review, 19 cases of EM were recorded in an 8-year period, and most of these cases were attributed to Mycoplasma pneumoniae infection (42.1%), HSV (5.26%), EBV (5.26%) and adenovirus (5.26%), without any mortality noted [6]. A similar pattern was also seen in a Swiss study of 42 cases of EM, where 30 cases were attributed to infections, 14 due to Mycoplasma pneumoniae, and six due to HSV infection [7].
Erythema multiforme usually resolves spontaneously in 3–5 weeks, but has a tendency to recur [2]. This is usually the case when it is found in association with HSV infection. In these cases, there is a role for aciclovir prophylaxis. Aciclovir use in EM is further discussed in the management section below.
References
1 Huff JC, Weston WL, Tonnesen MG et al. Erythema multiforme: a critical review of characteristics, diagnostic criteria, and causes. J Am Acad Dermatol 1983;8:763–75.
2 Lamoreux MR, Sternbach MR, Hsu WT. Erythema multiforme. Am Fam Physician 2006;74:1883–8.
3 Forman R, Koren G, Shear NH. Erythema multiforme, Stevens–Johnson syndrome and toxic epidermal necrolysis in children: a review of 10 years’ experience. Drug Saf 2002;25:965–72.
4 Von Hebra F. Atlas der Hautkrankheiten
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree