Rocky Mountain spotted fever
Aetiology and Pathology.
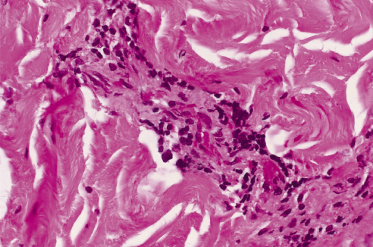
The causative organism of Rocky Mountain spotted fever (RMSF), R. rickettsii, is principally transmitted by the bite of ixodid ticks. Transmission by other means, such as exposure to infective tick tissues, as occurs in the process of crushing or removing ticks, is also presumed to occur. R. rickettsii has a tropism for endothelial cells. The pathological hallmark of RMSF is a lymphohistocytic vasculitis (Figs 61.1, 61.2). Endothelial damage occurs early in infection and results in innumerable minute foci of endothelial damage accompanied by haemorrhage and microthrombi as well as generalized increases in vascular permeability. These changes, in turn, lead to movement of plasma and plasma proteins into the interstitium. The net effect of this vascular injury is either focal (e.g. neurological or myocardial) or widespread organ damage.
Fig. 61.1 Photomicrograph of a skin biopsy from a patient with severe Rocky Mountain spotted fever, illustrating lymphohistiocytic vasculitis in the dermal vasculature.
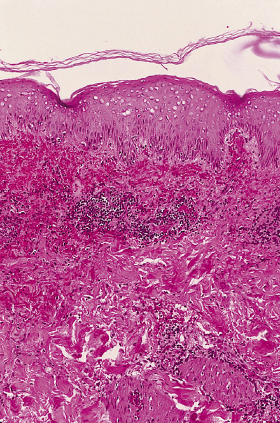
Fig. 61.2 Photomicrograph illustrating the intense infiltration of a dermal blood vessel by mononuclear cells in a patient with severe Rocky Mountain spotted fever.
Clinical Features.
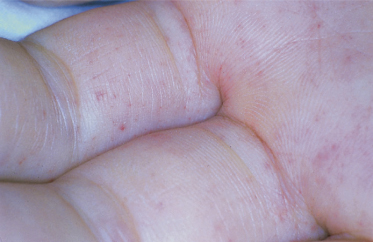
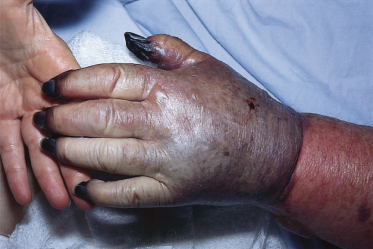
The highest incidence of RMSF occurs in children aged 5–9 years. The clinical features of RMSF in children and young adults are highly variable. Although, in general, illness is milder in children, individual cases may range in severity from mild to fulminant. Onset typically begins abruptly 2–12 days after tick bite or tick exposure. Headache, fever, chills, non-specific gastrointestinal complaints and myalgias are common symptoms early in the course of illness. Skin rash may begin as early as the first day of illness or as late as 7–10 days after onset, but in most cases rash appears between the third and fifth days of illness. In approximately 10% of cases, a skin rash never appears. However, cases in which skin rash is absent are not always mild in their course. In fact, cases in which the rash is fleeting, atypical in appearance or completely absent may have a fatal outcome [1]. In some cases, the rash may appear only a few hours before death [2]. In classic cases, the rash initially appears as a maculopapular eruption on the wrists and ankles and then becomes generalized (Fig. 61.3). As the illness progresses, the rash often becomes petechial and involves the palms and soles. In severe cases widespread cutaneous necrosis and even gangrene of the ears, scrotum and extremities may occur [3] (Fig. 61.4). Although a localized eschar is not a common finding in patients with RMSF, a localized necrotic lesion may appear at the site of tick bite and precede the appearance of generalized skin rash [4,5].
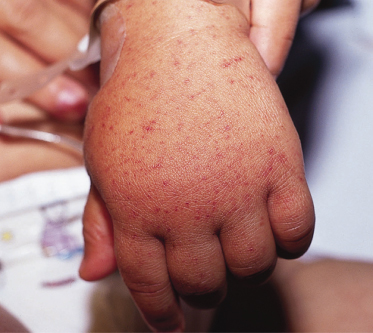
Periorbital oedema and oedema of the extremities may occasionally be a striking finding in children with RMSF (Fig. 61.5). Children with RMSF, particularly the very young, may not complain of headache (which is often a dominant symptom in adolescents and adults). Furthermore, gastrointestinal symptoms may be prominent in the early phases of illness in children.
Fig. 61.5 Child with Rocky Mountain spotted fever, who has oedema of the hands and a petechial rash.
Courtesy of Dr Neil Prose.
Prognosis.
Prognosis in RMSF is dependent upon both host factors and as yet poorly characterized virulence factors of the infecting organism [6]. Depending upon the geographical location, 20–80% of patients with RMSF who do not receive antirickettsial therapy will die of their illness. Disease is more severe in adults, males and individuals with glucose-6-phosphate dehydrogenase deficiency, and perhaps in alcoholics [7]. However, fulminant disease may occur in patients of both sexes and all ages and races. Delay in institution of antirickettsial therapy is an additional prognostic factor. Children treated with effective therapy within the first 5 days of onset have significantly better outcomes than those treated later [8]. Long-term sequelae such as mononeuropathy, neurogenic bladder and paralysis may occur in patients with severe disease [9].
Differential Diagnosis.
The initial clinical features of RMSF are so non-specific that, even in areas where the disease is endemic, the majority of children and adolescents with this disease are not correctly diagnosed at their first clinic visit [8]. RMSF is frequently mistaken for a wide array of other febrile illnesses including common viral exanthems and (early in illness) other viral illnesses without skin rash. Monocytic ehrlichiosis and infections due to Anaplasma phagocytophilium (previously called the agent of human granulocytic ehrlichiosis) may produce many or most of the symptoms of RMSF and also follow a recent tick bite, although patients with monocytic ehrlichiosis or human anaplasmosis are more likely to have leucopenia and less likely to have skin rashes than individuals with RMSF. Children with RMSF are often empirically treated with β-lactam antibiotics in the early phases of illness and then mistakenly thought to have a drug eruption when a skin rash appears a few days later. Gastrointestinal manifestations are often dominant in the early phases of illness; indeed, some children with RMSF have undergone appendectomies because of an erroneous initial diagnosis of appendicitis [10]. The gastrointestinal signs and symptoms may be particularly confusing in young children because they may not be able to articulate other clues to diagnosis, such as the presence of a recent tick bite, headache or myalgias.
Occasionally, children with RMSF present with unusual focal or generalized neurological symptoms that mimic viral meningoencephalitis or bacterial meningitis. Rarely, bizarre neurological symptoms such as hallucinations dominate the early presentation of illness, leading to misdiagnosis as psychiatric disease [11] or an intoxication. Severe cases of RMSF may mimic meningococcaemia to the point that even experienced clinicians cannot distinguish between the two diseases. In addition, RMSF has been confused with thrombotic thrombocytopenic purpura [12], streptococcal bacteraemia [13] and measles [14]. Occasionally, more than one child in a household will acquire RMSF nearly simultaneously, leading to the logical but mistaken assumption that the cause of illness is a common viral pathogen.
Diagnosis.
There is usually no definitive way to prove the diagnosis of RMSF in the early phases of illness; thus, treatment is typically and correctly administered on an empirical basis. Skin biopsy with demonstration of rickettsiae in tissue using immunofluorescent or immunoperoxidase staining is a useful test when it is positive, but a negative test does not exclude the diagnosis [15]. False-negative tests may occur in patients who have received antirickettsial therapy. Furthermore, skin biopsy is not a practical test in areas where reagents for immunofluorescent or immunoperoxidase testing are not readily available, in patients without a skin rash and in those who have received antirickettsial therapy for more than 24–36 hours. In most cases, the diagnosis is established by demonstrating a fourfold antibody titre rise using a microimmunofluorescent technique [16]. Rickettsiae can be cultured from both skin biopsy samples and blood. They can also be detected in blood or tissue by techniques utilizing polymerase chain reaction (PCR) technology [17]. However, PCR testing methods are neither widely available nor highly sensitive.
Treatment.
The only two drugs effective against R. rickettsii are tetracyclines and chloramphenicol. The latter is generally reserved for the treatment of pregnant women and for empirical treatment of severe cases in which meningococcaemia is thought to be a possible alternative diagnosis. Tetracycline (usually in the form of doxycycline) is the preferred treatment for most cases, in both adults and children. Although repeated doses of tetracycline can cause dental staining in children, a short course of therapy is unlikely to cause dental damage and doxycycline is considered to be the drug of choice, even in children [18]. Routine treatment of chloramphenicol to treat febrile children with a history of tick bite or suspected tick exposure would result in aplastic anaemia in one of every 25,000–40,000 treatment courses. Use of a tetracycline such as doxycyline is not associated with a risk of aplastic anaemia and the risk of dental staining is minimal to nil if therapy is discontinued 48–72 hours after defervescence.
The recommended dose of doxycycline for children is 200 mg/day in two divided doses for children greater than 45 kg in weight; children weighing less than 45 kg should receive 4.4 mg/kg bodyweight per day. The recommended dose of chloramphenicol for children is 25 mg/kg per day in four divided doses, up to a maximum dose of 2 g/day. Fluoroquinolones have been shown to have in vitro activity against spotted fever group rickettsiae, but they have not been adequately tested in humans with RMSF. Their use is generally unwise in children. Children with mild illness can usually be successfully treated as outpatients with oral doxycycline, but when severe disease, nausea or vomiting or altered mental status is present, intravenous therapy with either doxycycline or chloramphenicol and hospitalization is recommended.
References
1 Sexton DJ, Corey GR. Rocky Mountain ‘spotless’ and ‘almost spotless’ fever: a wolf in sheep’s clothing. Clin Infect Dis 1992;15:439–48.
2 Westerman EL. Rocky Mountain spotless fever: a dilemma for the clinician. Arch Intern Med 1982;142:1106–7.
3 Kirkland KB, Marcom PK, Sexton DJ et al. Rocky Mountain spotted fever complicated by gangrene: report of six cases and review. Clin Infect Dis 1993;16:629–34.
4 Walker DH, Gay RM, Valdes-Dapena M. The occurrence of eschars in Rocky Mountain spotted fever. J Am Acad Dermatol 1981;4:571–6.
5 Cox G, Sexton DJ. Eschar due to Rocky Mountain spotted fever. Clin Infect Dis 1995;21:315–19.
6 Walker DH. Rocky Mountain spotted fever: a disease in need of microbiological concern. Clin Microbiol Rev 1989;2:227–40.
7 Walker DH, Kirkman HN. Rocky Mountain spotted fever and deficiency in glucose-6-phosphate dehydrogenase. J Infect Dis 1980;142:771.
8 Kirkland KB, Wilkerson WE, Sexton DJ. Therapeutic delay and mortality in cases of Rocky Mountain spotted fever. Clin Infect Dis 1995;20:1118–21.
9 Archibald LK, Sexton DJ. Long-term sequelae of Rocky Mountain spotted fever. Clin Infect Dis 1995;20:1122–5.
10 Walker DH, Henderson FW, Hutchins GM. Rocky Mountain spotted fever. Mimicry of appendicitis or acute surgical abdomen? Am J Dis Child 1986;140:742–4.
11 Kirk JL, Sexton DJ, Fine DP et al. Rocky Mountain spotted fever: a clinical review based on 48 confirmed cases, 1943–86. Medicine 1990;69:35–45.
12 Turner RC, Chaplinski TJ, Adams GG. Rocky Mountain spotted fever presenting as thrombotic thrombocytopenic purpura. Am J Med 1986;81:153–7.
13 Milunski MR, Gallis HA, Fulkerson WJ. Staphylococcus aureus septicemia mimicking fulminant Rocky Mountain spotted fever. Am J Med 1990;83:801–3.
14 Nieburg PI, D’Angelo LJ, Herrmann KL. Measles in patients suspected of having Rocky Mountain spotted fever. JAMA 1980;244:808–9.
15 Walker DH, Cain BG, Olmstead PM. Specific diagnosis of Rocky Mountain spotted fever by immunofluorescent demonstration of Rickettsia rickettsii in cutaneous lesions. Am J Clin Pathol 1978;69:619–23.
16 Dumler JS, Walker DH. Diagnostic tests for Rocky Mountain spotted fever and other rickettsioses. Dermatol Clin 1994;12:25–36.
17 Sexton DJ, Kanj SS, Wilson K et al. The use of polymerase chain reaction as a diagnostic test for Rocky Mountain spotted fever. Am J Trop Med Hyg 1994;50:59–63.
18 Abramson JS, Givner LB. Should tetracycline be contraindicated for therapy of presumed Rocky Mountain spotted fever in children less than 9 years of age? Pediatrics 1990;86:123–4.
Other Spotted Fever Group Rickettsial Diseases
Aetiology and Pathology.
The pathogenesis and epidemiology of other tick-borne spotted fever group rickettsial infections are very similar to those of RMSF. Rickettsial pox, which is caused by R. akari, is unique in that it is transmitted by the mouse mite. Children typically acquire rickettsial pox when they are in locations where vermin control efforts have led to a reduction in the numbers of mice, resulting in the need for the mouse mite to find an alternative blood meal. The basic pathophysiology and pathobiology of other spotted fever group rickettsioses are similar to those seen with R. rickettsii infection.
Clinical Features.
Unlike RMSF, in which a localized eschar is quite rare, localized eschars occur at the site of the infecting tick bite in 20–70% of patients with R. conorii, R. australis, R. japonica and R. sibirica infection, and multiple eschars are usually present in patients with African tick bite fever (due to R. africae
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree