47 Diagnosis and Treatment of Cutaneous Malignancies
Introduction
Cutaneous malignancies, which are the most common cancers in the world, include melanoma and nonmelanoma skin cancers (NMSC), principally squamous cell carcinoma (SCC), and basal cell carcinoma (BCC). These tumors originate from the upper layer of the skin, called the epidermis. Many factors can play a role in the development of skin cancer, but exposure of the epidermis to ultraviolet (UV) radiation, principally from sunlight, is the most important factor. Less common cutaneous malignancies can arise from the deeper layers, the dermis and subcutaneous fat, or from other structures, such as hair follicles, sebaceous glands, eccrine glands, blood vessels, and nerves. Sometimes skin cancers are misdiagnosed, because they mimic benign growths that arise from these structures in the skin.
The incidence of skin cancer in the United States is approaching epidemic proportions. Currently, one in five Americans will develop a skin cancer during his or her lifetime. 1 There are now over 1 million cases of NMSC in the United States annually. 2 The incidence of melanoma continues to rise. It was estimated that 68,720 Americans will have been diagnosed as having melanoma in 2009, and the current lifetime risk for developing invasive melanoma is 1 in 58. 3 When the estimated 53,120 cases of in situ melanoma are added, the lifetime risk for being diagnosed as having melanoma is now 1 in 30. 4 It is postulated that the increase in incidence of skin cancer may be explained by changes in lifestyle or depletion of the ozone layer, which filters out the carcinogenic UV radiation from sunlight. 5 Epidemiologic studies suggest that an individual who develops a skin cancer is more likely to develop not only additional skin cancers, but other internal malignancies, such as lung cancer. 6 , 7 , 8 An individual who develops one NMSC has an estimated risk of developing one or more additional skin cancers of 35% at 3 years and 50% at 5 years. 9
In this chapter, we focus on the diagnosis and treatment of skin cancer, particularly SCC, BCC, and melanoma. The biology and histology of these neoplasms is included when relevant to management. Other more uncommon cutaneous malignancies are also reviewed.
Squamous Cell Carcinoma
Epidemiologic and Etiologic Factors
Cutaneous SCC is the second most common form of NMSC, with an estimated incidence of nearly 700,000 new cases annually in the United States. 10 The incidence of SCC, and all skin cancer, is increasing. SCC has been found to occur on all cutaneous surfaces, but most often occurs on the sunexposed areas of the head and neck. Cutaneous SCC is the second most common form of cancer in the white population. 11 The cause of SCC is multifactorial. Exposure to sunlight, fair skin, and reduced immunity are major risk factors. Certain environmental exposures and medical conditions are associated with an increased risk of SCC. When many predisposing elements are present in a patient, such as high cumulative sun exposure, fair skin, and immunosuppression after organ transplantation, the likelihood of developing SCC is extremely high.
The principal cause of SCC is UV radiation from sunlight. The majority of SCCs occur in elderly, fair-complexioned Caucasians who have had years of sun exposure. 11 Pigmentation plays an important role in protecting the skin from UV radiation; therefore, skin cancer is uncommon in individuals with dark pigmentation (Fitzpatrick classification IV–VI). UV-B–induced inactivation of p53, a key tumor-suppressor gene, occurs in nearly 90% of all SCC lesions and 75 to 80% of premalignant actinic keratosis (AKs). p53 plays an important role in cell cycle arrest at the G1/S checkpoint. 12 , 13 Both epidemiologic and experimental evidence points to UV-B as the major component in sunlight responsible for causing cutaneous SCC. 13 , 14 , 15
Clinical signs of chronic sun exposure that can be used to identify those at risk for SCC and skin cancer in general include freckling, telangiectasia, atrophy, rhytides, and precancerous lesions. 16 , 17 People who are engaged in an outdoor occupation or recreation, live close to the equator (where the sun is more intense), or are elderly (with more cumulative exposure to UV radiation) are at higher risk for the development of SCC. 18
Chemical exposure to organic hydrocarbons, pesticides, and arsenic (most commonly in contaminated well water) can be associated with the development of SCC. 19 , 20 , 21 Ionizing radiation can be associated with SCC. 22 The link between smoking and SCC development has been well established. 23 Certain genetic disorders and syndromes (e.g., xeroderma pigmentosum and albinism) are also associated with the development of SCC. 24 , 25 , 26 Chronic skin conditions, such as draining sinuses, burn scars, 27 ulceration, and infection, can also be associated with the development of SCC. 21
Impaired cell-mediated immunity (either drug- or diseaseinduced) is linked with the development of cutaneous SCC. Organ transplant patients requiring chronic immunosuppressant medications, such as cyclosporine, azathioprine, and prednisone, are at increased risk for SCC. T-cell depletion (particularly CD4? T cells) is associated with increased risk of skin cancer in longterm renal transplant recipients. 28 Organ transplant recipients have a 60- to 250-fold increased risk for cutaneous SCC and a 10-fold increased risk for BCC. 29 The incidence of malignancy increases with time after transplantation. One study showed the overall risk for a transplant patient to develop a first skin cancer increased from 10% after 10 years to 40% after 20 years of graft survival. 30 Cumulative incidence for the first NMSC rose linearly and without delay after transplant and was similar between SCC and BCC. 31 Development of SCC is also more common in diseases with impaired cell-mediated immunity, such as lymphoma or leukemia, 32 , 33 autoimmune disease, 34 and epidermodysplasia verruciformis (a rare hereditary immune deficiency in which human papillomavirus is associated with SCC). 35
Clinical Characteristics and Precursors
The most common precursor to SCC is the AK. AKs, also called solar keratosis, are sun-induced precancerous lesions. These scaly, circumscribed, rough, erythematous lesions are often better recognized by palpation than inspection. Independent risk factors for AKs include older age, male gender, cumulative sun exposure, and Fitzpatrick skin types I and II. 17 They are usually asymptomatic, but the patient may occasionally complain of itching or tenderness. Size varies from pinpoint size to large plaques, but they usually are 3 to 6 mm in diameter. AKs are important markers of UV-induced photodamage, and their presence generally signals an increased risk of NMSC. 36 Although AKs are biologically considered to be premalignant, histologically they appear to represent early SCC in situ. 37 About 20% of AKs will spontaneously involute. 38 However, the presence of AK is associated with a greater than sixfold increase in the risk of developing skin cancers. 39 Malignant conversion has been estimated to be on the order of a 1 in 1,000 per year and may involve p53 mutations. 40 , 41 Mutations of p53 have been identified in greater than 50% of premalignant AK lesions. 42
Another less common precursor to invasive SCC often associated with AKs is Bowen’s disease (SCC in situ). Histologically, one sees malignant cells filling the epidermis but not invading the dermis. Clinically, Bowen’s disease most often appears as a single erythematous, slightly scaly, minimally indurated patch that can be mistaken for eczema, fungus, or psoriasis. There can sometimes be multiple areas on a background of sun damage and AKs. The lesions measure from millimeters to centimeters. Bowen’s disease is most commonly found on the trunk. Any induration or hyperkeratotic thickening should alert the clinician to the possibility of invasive SCC in that area and a biopsy should be performed.
Invasive SCC typically presents on the sunexposed areas of the head, neck, and arms. They can arise de novo or from preexisting AKs or Bowen’s disease. Clinically, SCC most often presents as a hyperkeratotic growth or a crusted, indurated papule that on removal of the crust reveals a friable, red granular base. SCCs often have elevated and rolled edges with central ulceration. Pearliness and telangiectasia (found in BCC) are often absent. The tumor is firm, although more advanced cases can be soft and friable. Erosion and ulceration are more common with SCC than BCC. Histologically, SCC may show a wide spectrum of differentiation from cells showing complete keratinization (well-differentiated) to spindle-shaped cells that can only be distinguished from other tumors by special histological methods (poorly differentiated) ( Fig. 47.1 ). 12 Well-differentiated SCC is likely to present as a firm erythematous nodule of variable size, sometimes with an area of central hyperkeratosis ( Fig. 47.2 ). 12 Poorly differentiated SCC is more apt to present as a faintly erythematous nodule or plaque that is not well defined and in which erosion and ulceration is common.
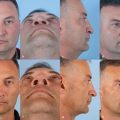
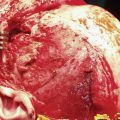
Keratoacanthoma has traditionally been thought to be a benign growth that resembles SCC clinically and histologically. The most unique clinical characteristic is its rapid growth. Over the period of a few weeks, the lesion can grow to several centimeters, then suddenly involute over several additional weeks. Classically, keratoacanthoma manifests as a smooth shiny papule or nodule with telangiectasia and a central keratotic plug, sometimes with ulceration. A keratoacanthoma may resemble a miniature volcano during the active growth phase. Unfortunately, the distinction of SCC from a solitary keratoacanthoma is often difficult. Expert dermatopathologists and clinicians regard solitary keratoacanthoma as a well-differentiated variant of SCC that deserves equivalent treatment. 43 , 44 , 45 One would be well advised to treat any keratoacanthoma in the same way as SCC because misdiagnosis could lead to inadequate treatment.
Biological Behavior and Risk Factors
In most cases, SCC is a locally growing neoplasm of sunexposed skin that is easily diagnosed and treated. In some cases, SCC may infiltrate the surrounding skin and adjacent structures, making treatment more difficult and recurrence after treatment more likely. If growth continues, metastasis may occur. Histology (differentiation, thickness, depth of invasion, perineural involvement), clinical size (diameter), etiology, immune status of the patient, and anatomical site of the tumor all have a role in predicting the risk of recurrence and metastasis ( Table 47.1 ). SCCs that recur locally are more likely to metastasize. 46 The most common route of spread for metastatic SCC is lymphatic.
Increased tumor thickness and depth of invasion are the most consistent histopathologic features of cutaneous SCC that recur and metastasize. Invasion into the deep (reticular) dermis, subcutaneous tissue, or below (Clark’s level IV and V) is strongly associated with the development of recurrence or metastasis. 47 , 48 , 49 , 50 In general, the more poorly differentiated the tumor, the higher the risk of recurrence and metastasis, but SCCs of any grade can metastasize. A particularly ominous sign is SCC with perineural invasion; there is a high risk of recurrence and metastasis, and direct intracranial extension along nerve fibers is a possibility. 46 , 51 , 52 , 53 Larger diameter lesions also have an increased likelihood of recurrence and metastasis.
Anatomical site appears to play an important role in behavior for SCC. Both the ear and the lip are consistently reported to have elevated recurrence and metastasis rates, as are the nasolabial creases, the periorbita, and the preauricular region. 46 Germane to the latter, direct invasion of the parotid gland by cutaneous SCC has been reported to have a 50% metastatic rate. 54
Conditions in which the SCC develops can also affect risk of recurrence and metastasis. SCCs arising in scars, on the edges of ulcers, and in skin damaged by ionizing radiation have substantial potential for metastasis. 46 SCC in organ transplant patients can be more aggressive because host immunosuppression can also increase the risk of metastasis. 55 , 56 An interesting cohort study out of Denmark that followed 83,896 patients found that unlike BCC, SCC is a marker of elevated all-cause mortality in an individual. The author postulated that SCC is associated with cumulative, prolonged UV exposure, which causes substantial systemic immune suppression. 57 , 58
Basal Cell Carcinoma
Epidemiologic and Etiologic Factors
BCC is the most common malignancy in humans. Estimates predict 1 million cases of NMSC in the United States and at least threefourths of these will be BCC. 2 Lifetime risk for a Caucasian developing a BCC has been estimated to be 28 to 33%. BCCs can be highly destructive but are almost always characterized by slow growth and by rare metastasis (< 1%). 59
BCCs arise from the basal keratinocytes of the epidermis and associated adnexal structures such as hair follicles. 60 Like SCCs, the most common factor involved in the pathogenesis of BCC is UV radiation, in particular UV-B radiation. In normal individuals, a long latency period of 20 to 50 years is typical between the time of UV damage and clinical onset of BCC. Intense, intermittent sun exposure has been implicated in the development of BCC (as opposed to chronic steady exposure with SCC). 61
Other factors associated with the development of BCC are the same as with SCC. These are environmental factors, such as ionizing radiation and arsenic exposure. Medical conditions associated with BCC development include immunosuppression (in organ transplant patients) and genetic disorders (e.g., nevoid basal cell carcinoma syndrome). 21 Nevoid basal cell carcinoma syndrome (also called basal cell nevus syndrome or Gorlin’s syndrome) is an autosomal dominant condition characterized by continued appearance of many BCCs at an early age. 62 Odontogenic keratocysts, palmoplantar pitting, intracranial calcification, and rib anomalies may also be seen with nevoid basal cell carcinoma syndrome. UV radiation–induced mutations in the human PATCHED gene have also been implicated in sporadic cases of BCC as well as nevoid basal cell carcinoma syndrome. 63 The extracellular hedgehog protein binds to a transmembrane receptor, patched homolog 1 (PTCH1), and prevents downstream PTCH1-mediated inhibition of signaling by smoothened homolog (SMO). SMO signaling activates a family of transcription factors. Usually inactive in adult tissues, mutations to either PTCH-1 or SMO result in constitutive activation of the hedgehog signaling pathway, thus allowing unrestricted proliferation of epidermal basal cells. 62
Clinical Characteristics and Precursors
There is no common clinical precursor to BCC as there is with SCC. BCC typically occurs on sunexposed areas, especially the head and neck, in particular the nose. Patients often present with a nonhealing “sore” of varying duration. Bleeding on slight injury is a common sign. Some BCCs heal spontaneously and form scar tissue as they extend. The lesions are sometimes itchy but often asymptomatic. There is gradual enlargement, with the tumor roughly doubling its 6-month volume at 1 year and quadrupling it at the end of 2 years. 64
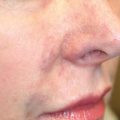
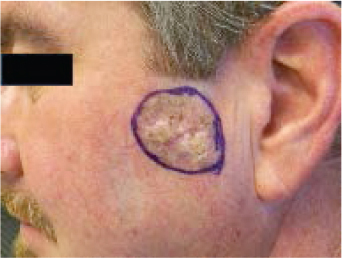
BCC can have a variety of clinical appearances, such as nodular, pigmented, cystic, or superficial. Nodular BCC is the most common variety and is usually composed of one or a few small, waxy, semitranslucent “pearly” papules or nodules that sometimes form around a central depression ( Fig. 47.3 ). 12 The tumor may be eroded, ulcerated, crusted, or bleeding. The trauma necessary for ulceration is usually slight, and patients frequently insist that the lesion is a scratch or razor nick. The edges of larger lesions have a characteristic rolled or raised border and are well circumscribed. Telangiectasias are visible coursing through the lesion. BCC may also be pigmented with brown or black pigmentation. Pigmented BCCs are seen more commonly in dark-skinned individuals and can mimic a mole or even a melanoma ( Fig. 47.4 ). 12 Cystic BCCs are filled with fluid that makes them look translucent blue-gray. These cystic nodules may mimic benign cystic lesions, especially around the eye where they may be confused with hidrocystomas. Superficial BCCs usually are pink to reddish brown scaly macules or plaques, seen occasionally with central clearing. Ulceration is less common than in nodular BCC. Superficial BCCs are more common on the trunk where they can be multiple. There is little tendency to become invasive. Superficial BCC may mimic psoriasis, fungus, or eczema. Occurrence of numerous superficial BCCs may be a clue to arsenic exposure.
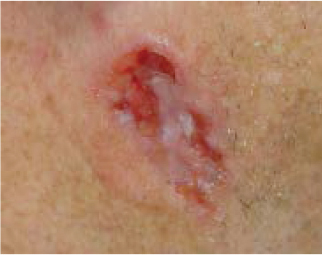
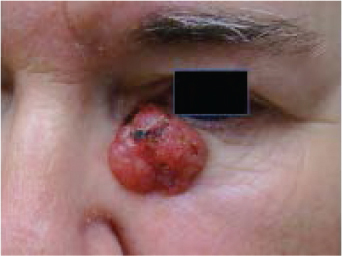
“Aggressive” BCCs can also have the appearance of pale, flat, smooth-surfaced plaques or macules that are typically ill defined but have deeper extension of tumor that cannot be detected clinically. They are also called “sclerosing” BCCs because they can masquerade as a scar. Telangiectasia, ulceration, erosion, scaling, and crusting are often absent. Clinically this tumor appears as a yellowish white, indurated plaque with illdefined borders. This is in contrast to nodular BCCs, which usually have welldefined borders. Oftentimes this deeper growth of aggressive BCC goes undetected for a long period and there is enormous destruction of normal tissue by the time the tumor is discovered. This hidden growth is referred to as subclinical extension. Subclinical extension refers to the portion of a tumor that is present histologically but cannot be detected clinically. Even if the border is seemingly well defined, a tumor can invisibly extend far beyond clinically apparent margins. BCCs with subclinical extension may appear yellowish white when the skin is stretched and be firm to the touch. Significant subclinical extension is most commonly seen with the morpheaform, infiltrating, and micronodular histologic subtypes—discussed in the following section.
When examining possible skin cancers, it is best to use good lighting and magnification. The affected skin should be stretched, squeezed, and palpated to better estimate the tumor size and depth. Oblique illumination of the tumor can highlight surface changes, such as a “rolled” border. A gentian violet pen can be used to mark the clinical margin of the tumor. Occasionally, the true extent of the tumor can be grossly underestimated because of subclinical extension.
Biological Behavior and Risk Factors
Like SCC, BCC is a locally growing neoplasm that is usually easily diagnosed and treated. In some cases, BCC may infiltrate the surrounding skin and adjacent structures. Although the clinical appearance of the BCC can alert the clinician to the possibility of subclinical extension, the histological appearance of the BCC obtained from a generous skin biopsy (see “Biopsy Techniques” discussed under “Melanoma”) can more accurately predict which BCC will have a greater chance of incomplete surgical removal (and subsequent recurrence). 65 The micronodular, infiltrative, and morpheaform histological subtypes of BCC can be more difficult to detect clinically and to eradicate than the nodular histological subtype of BCC because of the patterns of growth within the skin ( Fig. 47.5 ). 12 Micronodular, infiltrative, and morpheaform BCCs grow in a dispersed pattern, enlarging beneath the epidermis by small, diffuse fingerlike extensions that can permeate through tissue without much displacement of normal tissue. Tumors become infiltrative when basaloid islands lose their epidermal connections and invade the underlying dermis. Infiltrative growth and perineural invasion portends a more aggressive clinical behavior and increased risk of recurrence. 65 Extension from the epidermis is minimal so that there is little surface change to reveal the tumor during skin examination. In contrast, nodular BCCs have an aggregated pattern that enlarges in a circumscribed expansile manner, displacing more tissue and becoming more raised and clinically evident with time. BCC of any histological subtype can have malignant extension that is invisible to the eye, but micronodular, infiltrative, and morpheaform types are consistently deceptive and actually much larger than they appear clinically in comparison with nodular BCC. 66 , 67 , 68 , 69
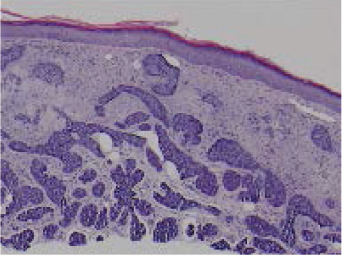
Micronodular, infiltrative, and morpheaform BCCs often need larger excision margins to effect complete removal of these subtypes. 70 The invisible portion of tumor may invade deeply into subcutaneous tissue, bone, and cartilage, causing extensive local destruction and mutilation. 71 , 72
Metastatic BCC is rare but more cases are being reported. 73 , 74 , 75 BCCs that metastasize have almost always been subjected to repeated incomplete excisions or represent lesions that have been so neglected as to reach extremely large size without the benefit of treatment. 76 A BCC that metastasizes has an extremely poor prognosis; according to the English literature, the median survival of patients with metastatic BCC is 10 months. 73
Treatment Options for SCC and BCC
Suspicious lesions deserve complete skin examination and an assessment of the regional lymph nodes. In addition to skin biopsy, imaging studies may be indicated for suspicion of deep structural involvement such as bone, perineural disease, and deep soft tissue, or clinical concern for regional lymph node involvement. With perineural suspicion, magnetic resonance imaging is preferred. Palpable lymph nodes or those identified on imaging studies require fine needle aspiration to determine regional disease status.
The choice of therapeutic modalities is similar for SCC and BCC ( Table 47.2 ). The National Comprehensive Cancer Network categorizes BCC and SCC tumors into low-risk and high-risk recurrence subgroups that inform treatment choices. In general, risk stratification is based on tumor size, tumor borders, primary versus recurrent status, RT history, immunosuppression, histologic subtype, perineural involvement, and depth and differentiation (for SCC) ( Table 47.2 ). Careful consideration of the options should be undertaken—especially with SCC, given the greater potential for morbidity and mortality. Standard treatment can be divided into excision and field therapy.
For the majority of BCC and SCC cases, those with at least one National Comprehensive Cancer Network risk factor, but without deep structural involvement or regional disease, Mohs micrographic excision (MME) is a preferred treatment. This method uses horizontal frozen sections and intraoperative tumor mapping to provide a higher cure rate for SCC and BCC than standard excision while leaving a smaller wound. When compared with other modalities, MME obtains higher cure rates—as high as 99% for primary BCC and SCC. 46 , 77 The reason for the high cure rate is that 100% of the surgical margin (deep and peripheral) is examined microscopically in combination with precise mapping of the surgical site and histologic specimen.
At the beginning of MME, the skin is anesthetized and the tumor is excised just beyond the clinical margin. The tissue is frozen and very thin sections are mounted on microscope slides. The tissue is oriented so that the sections are made horizontally across the deep and lateral margins of the specimen instead of vertically (the usual method). The dermatopathologist then reads these sections, looking for remaining tumor cells by examining the entire periphery. By mapping the tumor site, the surgeon can pinpoint the location of residual disease and remove only the area that contains cancerous tissue. One or more layers may need to be excised and inspected before all residual cancer cells are found. A more detailed description can be found elsewhere. 78
MME should be very seriously considered for SCCs or BCCs that are recurrent, large (greater than 1 cm), or that have indistinct margins or are in critical locations. A critical location may be defined as one where tissue sparing is paramount (e.g., nasal tip, eyelid, concha) or recurrence is common (e.g., nose, ear, periocular). MME should also be considered for the more difficult histologic patterns, such as poorly differentiated SCC or BCC with morpheaform; infiltrative, micronodular, or mixed morphology; or tumors with perineural involvement. If positive margins are reported after standard excision with no obvious clinical tumor, then MME should be considered. The need for MME increases when several these factors are present (e.g., recurrence, infiltrative histology, perineural invasion, etc.).
MME offers the lowest 5-year recurrence rates of all treatment options: primary lesions (1–2%) and secondary lesions (4–7%). 79 Multiple reviews of the literature support MME as a more costeffective treatment of NMSC (including BCC) than standard excision. 80 , 81
Wide local excision with postoperative margin assessment for SCCs and BCCs remains an acceptable therapeutic choice particularly for low-risk tumors, those without any high-risk features, 82 or for those tumors with risk features in a geographic region wherein MME is unavailable. A standard excision is usually done under local anesthetic, and the tumor is removed with a margin of clinically normal-appearing skin. Selected vertical sections (either frozen or permanent) from the specimen are then examined microscopically to determine if margins are free of tumor. Unfortunately, this method samples less than 1% of the true surgical margin, leaving the possibility that the examined sections may not include areas in the specimen where there are tumor extensions. Despite this disadvantage, cure rates greater than 90% can be obtained in many cases with standard excision for SCC and BCC. 46 , 83 Although it is potentially dangerous to assign a “standard” margin, current surgical recommendations call for margins of 4 mm of healthy tissue in excision of nodular BCC and more than 7 mm in aggressive BCC. 66 , 67 , 69 , 84 For SCC, one study proposed minimal margins of excision of 4 to 6 mm around the clinical borders. 85
Those tumors with deep structural disease require radical resection with margin control and reconstruction. Special consideration is given to patients with very high-risk SCC tumors without clinical or radiographic evidence of regional disease. Recent series have investigated the merit of sentinel lymph node biopsy (SLNB) for patients with factors such as depth > 6 mm or beyond subcutaneous fat, > 2 cm diameter on ear or hairbearing lip, or some of combination perineural invasion, poor differentiation, and immunosuppression; however, the value of SLNB in the high-risk cutaneous SCC group has yet to be established. Positive fine needle aspiration or SLNB warrants regional lymph node dissection and/or parotidectomy with adjuvant radiation therapy (RT) considered for positive nodes, and concomitant RT plus chemotherapy considered for more than two positive nodes, or incompletely excised nodal disease.
Field therapies destroy tissue in an area within and around the tumor, and the tissue is not evaluated histologically for margins. Commonly used field therapies include RT, cryosurgery, imiquimod topical application, and curettage and electrodesiccation.
Cryosurgery, a field therapy, destroys tissue by freezing (usually with liquid nitrogen) to a tumoricidal temperature. In most cases, cryosurgery is best limited to small, superficial SCCs and BCCs. In skilled hands, cryosurgery will give a cure rate in excess of 95% on selected SCCs and BCCs. 86 Wounds heal with secondary intention and leave hypopigmented, soft, sometimes depressed scars.
Curettage followed by electrosurgery is indicated only for the smallest, most superficial, and well-circumscribed SCCs and BCCs. The curette has a sharp edge that scrapes away soft tumor tissue followed by electrodesiccation of the wound base. The cosmetic appearance is usually minimally satisfactory. In one study, for sites at low risk for recurrence (neck, trunk, and extremities), BCCs of all diameters responded well to curettage and electrodesiccation, with an overall 5-year recurrence rate of 3.3%. 87
Topical imiquimod acts as an agonist of the toll-like receptor 7, which activates the cellular immune response to destroy dysplastic keratinocytes. Imiquimod 5% is approved by the U.S. Food and Drug Administration (FDA) to treat nonfacial biopsy-confirmed superficial BCC. 88 Although the use of topical therapy does not confirm margin control, imiquimod monotherapy has shown a 5-year clearance rate as high as 80%.
RT, another field therapy, can be used as a primary therapeutic alternative or as an adjunctive therapy to surgery. 89 RT is a proven field therapy that achieves cure rates in excess of 90% for appropriately selected patients with SCC and BCC. It is an excellent choice for patients who are not good surgical candidates or who do not wish to have surgery. 90
Vismodegib is the first Hedgehog pathway inhibitor to be approved in the United States. It is indicated for the treatment metastatic BCC, locally advanced BCC that has recurred after surgery, or in patients who are not candidates for surgery or radiation. In an ongoing, noncomparative, phase II trial, oral vismodegib was effective in treating patients with locally advanced or metastatic BCC with an acceptable tolerability profile. 91
In summary, SCCs and BCCs are the most commonly encountered skin cancers in the world. Clinicians should be familiar with their clinical appearance so that diagnosis and treatment can be initiated early. When treatment is delayed, significant morbidity and mortality can ensue. Many therapies obtain reasonable cure rates, but MME remains the gold standard.
Melanoma
Epidemiological and Etiologic Factors
Melanoma is a malignancy arising from melanocytes, the pigment-producing cells of the skin. In the year 2012 in the United States there were 76,250 new diagnoses with concurrent 9,180 deaths expected because of melanoma alone. 92 Melanoma is steadily increasing among whites with a 60% increase observed within the last 30 years. These data quote melanoma as the fifth and seventh most common new diagnosis of malignancy among men and women, respectively. 93
Usually, melanoma arises in sunexposed areas. During the 1970s, cutaneous melanoma was mainly observed on the trunk of males and extremities of females. However, most likely because of changes in lifestyle habits and clothing, current reports state lesions are most common on the chests of females and lower extremities of males. 94
The gene BRAF (V600E) is thought to be crucial to the pathogenesis of melanoma. Several studies implicate that a single mutation in this gene is observed in 50 to 60% of cutaneous melanomas. 95 These mutations are often associated with younger patients (86% between 20 and 30 years old), a predilection for the trunk and extremities, high nevus counts, few freckles, Fitzpatrick skin type I or II, and the superficial spreading subtype. 96
There are environmental exposures that play an important role in melanoma. Sun exposure is the most important environmental risk and the only one that remains potentially modifiable. It is of interest that people who have been exposed to the sun on a regular basis appear not to be at increased risk of melanoma. Persons at highest risk for melanoma are those with fair complexions who have had intermittent sun exposure and severe sunburns. In particular, the skin type most associated with melanoma is a Celtic complexion, including pale skin, freckles, blond or reddish hair, and light eyes. Melanoma is uncommon in Caucasians with dark skin and even more uncommon in people of Asian or African American background.
Artificial UV exposure by tanning is an additional risk factor. This source of intermittent radiation became popular 30 years ago and emits UV-A and UV-B radiation. UV-B rays are the primary factor in the carcinogenesis of skin cancer; however, individuals must remember that UV-A can also be absorbed by melanocytes. Artificial sources produce very high levels of UV-A radiation. An analysis of artificial sources concluded that an increased risk (relative risk, 1.15) existed for any individual that had ever used a tanning bed, and even higher was observed (relative risk, 1.75) if this occurred before the age of 35. 97
Clinical Characteristics
Many melanomas arise from preexisting nevi, though most probably arise de novo. Usually they have irregular borders, are raised above the skin surface, and contain color variation, though occasionally they are homogenously black. A mnemonic useful for identifying melanomas is: A (asymmetry), B (border irregularity), C (color variation), D (diameter > 6 mm), E (elevation). All of these features are evident in the melanoma shown in ( Fig. 47.6 ). 98 Realizing, however, that the goal is to diagnose melanoma early, small and flat lesions that satisfy the A-B-C criteria or that are totally black should be considered suspicious, such as the dysplastic nevus containing a 3-mm melanoma with 0.8 mm tumor thickness.
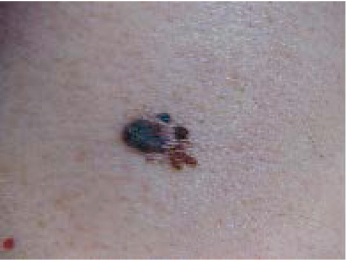
Unfortunately, some melanomas do not have brown or black pigment and look very much like normal skin. These can be very difficult to diagnose, even by an experienced physician. Among these amelanotic melanomas, there are a disproportionate number that fit into an uncommon histological group of desmoplastic melanoma. 99 In practice, given the variable presentations of melanoma, any pigmented skin lesion that has changed should be biopsied.
Though melanoma arises in defined cutaneous sites in ∼ 90% of cases, it can also arise from mucous membranes, from the eye, from unknown sites, or, rarely, from viscera. These presentations are uncommon but well described, and familiarity with each is important in the management of patients with melanoma.
Approximately 1 to 2% of melanomas arise from mucous membrane sites, and these are distributed equally among three different body areas: the mucous membranes of the head and neck (oropharynx, nasopharynx, and sinuses), the anorectal region, and the female genital tract. 100 These lesions are usually large when diagnosed because they are in areas not readily visible to the patient or to family. They are usually associated with a very poor prognosis. 101 Though up to 10 to 20% of patients may be alive at 5 years, the vast majority of patients will ultimately succumb to the disease. Radical resection does not appear to improve patient survival, compared with wide excision, but it may improve locoregional control. 102
Ocular melanomas account for 2 to 5% of melanomas, and they may arise from the retina, the iris, the ciliary body, or other sites in the eye. These melanomas are often identified as small lesions as they may be visible or may affect vision. Often they can be treated by radiation, but advanced lesions require enucleation. Though these tumors share many phenotypic features with cutaneous melanomas, they behave in a distinct pattern. Nodal metastasis is rarely seen, presumably because of the lack of ocular lymphatics. 103 Instead, the first site of metastasis is almost always the liver. In many cases, it is the only site of metastasis and the liver metastasis is the cause of death.
In 5 to 10% of patients who present with metastatic melanoma, no primary melanoma can be identified. These most commonly present as melanoma in a lymph node or in cutaneous sites. 104 In some cases, a history of a pigmented lesion that spontaneously regressed can be elicited. In some cases, it is believed that the immune system may have destroyed the primary melanoma. Another hypothesis for melanoma in a lymph node without a known primary is that the melanoma may have arisen there primarily, as isolated nests of nevus cells can be found incidentally in some lymph nodes. However, most cases remain unexplained.
Biologic
Most melanomas go through two growth phases, the first being a radial growth phase (RGP), when the tumor cells proliferate at the dermal–epidermal junction and the tumor expands radially. This may include lesions confined to the epidermis and those with superficial dermal involvement ( Fig. 47.7 ). 98 Subsequently, the lesion begins to invade more deeply into the dermis, with expansile nests of cells growing vertically. This is often associated with the development of a palpable nodule in the lesion, and this is considered the vertical growth phase (VGP), the portion of the tumor associated with metastatic risk. Depth into the dermis portends greater access for cancer cells to lymphatic channels and vasculature. Melanomas typically metastasize through lymphatic channels, usually to the regional nodes draining the primary melanoma, or through hematogenous dissemination to distant sites.
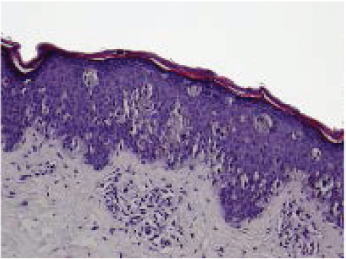
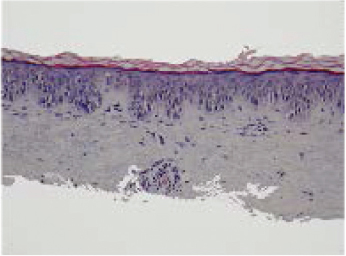
A common presentation of melanoma in the head and neck region is lentigo maligna ( Figs. 47.8 and 47.9 ), 98 which is a form of melanoma in situ. In some cases, a desmoplastic melanoma may arise from a lentigo maligna, a process illustrative of the RGP to VGP transition. Similar patterns of radial and vertical growth also occur with superficial spreading melanomas and acral lentiginous melanomas. The exception to this sequence of growth phases involves the nodular type of melanoma, which only has a VGP. 98 Thin melanomas excised while still in the RGP have little metastatic potential and excellent prognosis. 105 , 106 , 107
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree