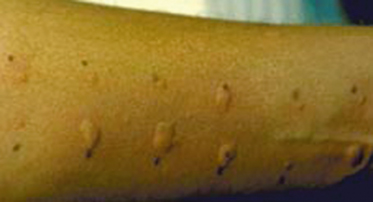
Feeding aversion/feed refusal
Dysphagia
Diarrhoea
Constipation (with/without perianal rash)
Abdominal pain
Blood in stool
Iron deficiency anaemia
Angio-oedema
Urticaria
Recurrent otitis media
Chronic cough
Wheezing
In infants with moderate-to-severe eczema, the relationship between milk ingestion and the development of eczema may or may not be obvious. Certainly a high index of suspicion is required when evaluating any infant with the combination of infantile eczema and features of altered gut motility (colic, reflux, persistent crying, feeding aversion). The absence of diarrhoea or growth failure does not preclude the diagnosis of cow’s milk allergy. Furthermore, it should be recognized that infants can react to minute amounts of milk and other food proteins transmitted within their mother’s breast milk. These proteins are capable of driving allergic inflammation in both the skin (eczema) and gut (e.g. diarrhoea) [14,16,17]. Maternal dietary exclusions may be justified if symptoms are attributed to maternal ingestion but they are no longer advised as a preventive strategy [18].
A clinical phenotype of severe eczema, multiple food allergies, failure to thrive, diarrhoea, hypoalbuminaemia and protein-losing enteropathy has been described in breast- and bottlefed infants at around 3–6 months of age. The clinical features can be confused with Netherton syndrome or severe immunodeficiency syndromes (severe combined immunodeficiency, Omenn syndrome), particularly as serum immunogloblins (IgG, A and M) are invariably low, secondary to protein loss in the gut. Response to an extensively hydrolysed formula is poor. Most infants respond dramatically to the removal of causative food proteins from the infant diet and institution of amino acid-based feeds [19].
References
1 Johansson S, Hourihane J, Bousquet J et al. A revised nomenclature for allergy. An EAACI position statement from the EAACI Nomenclature Task Force. Allergy 2001;56(9):813–24.
2 Muraro A, Roberts G, Clark A et al., for the EAACI Task Force on Anaphylaxis in Children. The management of anaphylaxis in childhood: position paper of the European Academy of Allergology and Clinical Immunology. Allergy 2007;62(8):857–71.
3 Sampson HA, Munoz-Furlong A, Campbell RL et al. Second symposium on the definition and management of anaphylaxis: summary report – Second National Institute of Allergy and Infectious Disease/Food Allergy and Anaphylaxis Network symposium. J Allergy Clin Immunol 2006;117(2):391–7.
4 Bock SA, Atkins FM. Patterns of food hypersensitivity during sixteen years of double-blind, placebo-controlled food challenges. J Pediatr 1990;117(4):561–7.
5 Flinterman AE, Knol EF, Lencer DA et al. Peanut epitopes for IgE and IgG4 in peanut-sensitized children in relation to severity of peanut allergy. J Allergy Clin Immunol 2008;121(3):737–43, e710.
6 Sampson H. Role of immediate food hypersensitivity in the pathogenesis of atopic dermatitis. J Allergy Clin Immunol 1983;71(5):473–80.
7 Sampson H, McCaskill C. Food hypersensitivity and atopic dermatitis: evaluation of 113 patients. J Pediatr 1985;107(5):669–75.
8 Worm M, Ehlers I, Sterry W, Zuberbier T. Clinical relevance of food additives in adult patients with atopic dermatitis. Clin Exp Allergy 2000;30:407–14.
9 Ehlers I, Worm M, Sterry W, Zuberbier T. Sugar is not an aggravating factor in atopic dermatitis. Acta Dermatol Venereol 2001;81:282–4.
10 Niggemann B, Sielaff B, Beyer K, Binder C, Wahn U. Outcome of double-blind, placebo-controlled food challenge tests in 107 children with atopic dermatitis. Clin Exp Allergy 1999;29:91–6.
11 Celik-Bilgili S, Mehl A, Verstege A et al. The predictive value of specific immunoglobulin E levels in serum for the outcome of oral food challenges. Clin Exp Allergy 2005;35(3):268–73.
12 Breuer K, Heratizadeh A, Wulf A et al. Late eczematous reactions to food in children with atopic dermatitis. Clin Exp Allergy 2004;34(5):817–24.
13 Hill D, Firer J, Shelton M, Hosking C. Manifestations of milk allergy in infancy: clinical and immunologic findings. J Pediatr 1986;109(2):270–6.
14 Host A, Husby S, Osterballe O. A prospective study of cow’s milk allergy in exclusively breast-fed infants. Incidence, pathogenetic role of early inadvertent exposure to cow’s milk formula, and characterization of bovine milk protein in human milk. Acta Paediatr Scand 1988;77(5):663–70.
15 Vanderhoof J, Murray N, Kaufman S et al. Intolerance to protein hydrolysate infant formulas: an underrecognized cause of gastrointestinal symptoms in infants. J Pediatr 1997;131(5):741–4.
16 Wilson N, Self T, Hamburger R. Severe cow’s milk induced colitis in an exclusively breast-fed neonate. Case report and clinical review of cow’s milk allergy. Clin Pediatr (Phila) 1990;29(2):77–80.
17 Jarvinen KM, Suomalainen H. Development of cow’s milk allergy in breast-fed infants. Clin Exp Allergy 2001;31(7):978–87.
18 Greer FR, Sicherer S, Burks AW. American Academy of Pediatrics Committee on Nutrition; American Academy of Pediatrics Section on Allergy and Immunology. Effects of early nutritional interventions on the development of atopic disease in infants and children: the role of maternal dietary restriction, breastfeeding, timing of introduction of complementary foods, and hydrolyzed formulas. Pediatrics 2008;121(1):183–91.
19 Hill DJ, Cameron D, Francis DE, Gonzalez-Andaya AM, Hosking CS. Challenge confirmation of late-onset reactions to extensively hydrolyzed formulas in infants with multiple food protein intolerance. Allergy Clin Immunol 1995;96(3):386–94.
The Diagnosis of Food Allergy in Patients with Eczema
An accurate diagnosis of food allergy is important as it allows for a targeted approach to allergen avoidance and the implementation of an appropriate management plan. Conversely, a negative allergy diagnosis allows for relaxation of dietary restrictions, which are often erroneously imposed on children in the absence of a specific allergy diagnosis [1].
The gold standard test for food allergy diagnosis is the DBPCFC. These tests are time-consuming and expensive and not accessible to most patients outside research studies. The diagnosis therefore needs to rely on a stepwise approach which includes a detailed allergy-focused history and food-specific allergy tests which are interpreted within the context of the allergy history. When there is still doubt about the diagnosis of food allergy, oral provocations tests are required after a trial period of dietary elimination.
The History
A detailed allergy-focused history should focus on the following areas.
Infant Feeding
A history of breast versus formula feeding, detailing the time period of exclusive breastfeeding. The relationship of eczema onset to the introduction of formula feeds. The type of formula feed and the infant’s acceptance thereof. The onset of severe eczema during a period of exclusive breastfeeding may be secondary to food proteins excreted within breast milk which are capable of causing adverse reactions [2].
Gastrointestinal Symptoms
This should explore the presence of gut dysmotility in infancy as well as a current history of colic, abdominal pain, vomiting, reflux, feeding aversion, diarrhoea, constipation, blood or mucus in stools and failure to thrive. The presence of gastrointestinal symptoms in a patient with eczema should raise awareness of the possibility of food allergy.
History of Immediate Reactions to Specific Foods (Past or Present)
A history of type 1 reactions to foods is important to ascertain. This should explore the time of onset in relation to ingestion, the quantity of food required to cause a reaction, any previous reaction or prior tolerance of that food and whether the reaction was of sufficient severity to cause anaphylaxis. As the natural history is for children to acquire tolerance to food allergens over time, it is necessary to determine reactions to foods in infancy as well as current reactions to establish a meaningful picture of the child’s allergic status.
A useful approach is to establish whether the patient is able to ingest a normal portion size of common food allergens in order to ascertain oral tolerance, e.g. a healthy 6 year old should be able to tolerate a glass of milk, whole egg, slice of bread, peanut butter sandwich, piece of fish, tablespoon of pulses, seeds on bread and one kiwi fruit. A history of food aversion may be due to dislike of a particular food or an underlying food allergy. Young children with egg allergy frequently refuse to eat egg but receive small amounts of heated or baked egg within food products, e.g. cakes. In response to the question ‘Does your child eat egg?’, parents will frequently reply ‘yes’ as they are aware of their child’s ingestion of certain egg products but fail to recognize the possible importance of their child’s refusal to eat scrambled egg or raw egg within mayonnaise.
History of Eczematous or Gastrointestinal Reactions to Specific Foods (Past or Present)
Is there a history of foods causing an eczematous flare or onset of gastrointestinal symptoms? A history of food causing an eczematous flare in patients with persistent eczema is frequently absent. This has been shown in many clinical investigations. In a placebo-controlled study which demonstrated a 60% improvement in eczema patients adhering to a milk- and egg-free elimination diet, there was no correlation between the parents’ suggestions that milk and/or eggs triggered their child’s eczema [3]. Retrospective analyses by Niggemann & Breuer have shown that the patients’ history of food-related eczema does not have a high diagnostic specificity [4,5].
Current Diet and Prior History of Tolerance to Foods
Which of the main allergenic foods are included within the current diet? Are these foods present in normal portion sizes? Has there been any previous attempt to eliminate foods from the patient’s diet? Was this done with any specialist dietetic supervision? Up to 75% of children with eczema have undergone some form of dietary exclusion, usually without the supervision of a health professional or dietitian [6]. Unsupervised dietary elimination is potentially hazardous and can cause iron deficiency anaemia, rickets and symptoms associated with deficiency of vitamins A, C, B, zinc and selenium [7]. It is therefore important to establish the nutritional adequacy of the patient’s current diet as well as any proposed elimination diet, ensuring that they are meeting their calcium, vitamin, mineral and protein requirements.
Could Cross-Reacting Allergens Be Relevant?
Certain foods display a high degree of allergen cross-reactivity which may be clinically relevant, e.g. the association between peanut allergy and allergy to sesame and tree nuts. In the absence of a history of oral tolerance to cross-reacting allergens, allergy testing is indicated to screen for potential, clinically relevant cross-reactivity.
Age of Onset of Eczema
Eczema onset in infancy is far more likely to be associated with food allergy than eczema onset in a child >5 years [8]. A history of late eczema onset can therefore be helpful in ‘ruling out’ the possibility of allergy to certain foods (milk, soya, egg, wheat) previously tolerated in good amounts in infancy.
Eczema Severity
The probability of food allergy is greater in younger children and infants and those with severe disease. This is particularly true in infancy where the prevalence of food allergy ranges from 10% to 65% depending on infant severity [8]. When associated with symptoms of gut dysmotility, the association between food allergy and eczema is strengthened.
Co-Morbid Associations
Asthma is a specific risk factor for anaphylaxis particularly within the context of poor asthma control [9]. It is important to be aware of a patient’s asthma status prior to embarking on elimination diets and challenge testing within the context of positive allergy-specific IgE tests to foods. Patients with chronic asthma and co-existing or suspected food allergy are best managed by specialists with allergy training.
Family History of Atopy
The risk of atopy increases if a parent or sibling has atopic disease (20–40% and 25–35%, respectively), and is higher still if both parents are atopic (40–60%) [10].
Allergy-Specific Tests
Available tests for the diagnosis of food allergy include skinprick tests (SPT), specific IgE tests (sp IgE) and the atopy patch test (APT). None of these tests, however, confirms or refutes the diagnosis of food allergy in the absence of an individual patient history which seeks to establish the prior probability of the allergen being causal. In order to minimize the number of false-positive tests, the selection of candidate allergens for testing should not be open-ended and should include relevant allergens based on the clinical history, patient’s age, allergic condition and geographical location. In the absence of a history of food allergy or prior exposure to a food, it may be necessary to select a screening panel of relevant food allergens, e.g. for an infant this would typically include cow’s milk, hen’s egg, wheat, soya, fish (cod or salmon), peanut and sesame.
When applying normal ranges for test positivity (i.e. SPT wheal diameter of 3 mm or greater and/or specific IgE >0.35 KU/L), allergy tests show good sensitivity and negative predictive value (generally >90%) but moderate specificity and PPV (30–50%) in identifying a food hypersensitivity reaction. A negative test, in the presence of a good response to a histamine positive control, is therefore good at ruling out an IgE-mediated reaction whereas a positive test will overestimate IgE-mediated allergy 50% of the time if used as a screening test (Fig. 31.1). When the index of suspicion is high, the tests are useful for confirming allergy and conversely when the index of suspicion is low, the tests are useful for ruling out a diagnosis of allergy. When there is a lack of correlation between the history and tests of specific IgE or when the history and tests are equivocal, confirmation by way of an open or blinded provocative challenge test is usually required to reach the diagnosis [11,12].
The specificity of a SPT can be increased by utilizing fresh foods for testing [13] and by raising the wheal diameter considered positive [14].
Changing the parameters for sIGE positivity to certain allergens (milk, egg, cod, peanut) but not others (wheat, soya) can similarly increase the specificity and performance of allergy tests [15] (Table 31.4). The larger the SPT mean wheal diameter, or quantity of sIgE, the more likely that the child has clinical allergy as opposed to sensitization. This relationship has allowed the development of predictive diagnostic cut-off values, which have been validated in specific patient populations for selected foods. These diagnostic cut-off values represent the SPT diameter or sIgE value at which a certain proportion (usually 95%) of patients were demonstrated as having proven (by DBPCFC) clinical allergy. Published 95% PPVs are, however, population specific and may vary in different populations [16–18]. There are no decision points yet for eczematous reactions to foods and these PPVs have not been calculated specific to an eczematous population. These published PPV values may therefore serve as a guide only when making decisions on whether oral food provocation challenge test is needed.
Table 31.4 Positive predictive values for food-specific IgE and skinprick tests
| Allergen | 95% PPV |
| 95% specific IgE levels (U/mL) positive predictive values | |
| Egg | 7 |
| Infants <2 years | 2 |
| Milk | 15 |
| Infants <2 years | 5 |
| Peanut | 15 |
| Tree nut | 15 |
| Fish | 20 |
| 95% skinprick tests (wheal diameter in mm) positive predictive values | |
| Milk | 8 |
| Infants <2 years | 6 |
| Egg | 7 |
| Infants <2 years | 5 |
| Peanut | 8 |
| Infants <2 years | 4 |
Niggemann et al. analysed whether utilizing the calculated ratio of sIgE/total IgE versus sIgE alone would improve the diagnostic accuracy of sIgE tests in the diagnosis of food allergy in eczema patients with serum total IgE levels of 0.3 to 13,525 Ku/L (median 94.3 Ku/L). Receiver operator characteristics (ROC) curves were performed and predicted probabilities and predictive decision points were calculated based on the retrospective analysis of 992 DBPCFC performed in 501 children (median age 13 months). The calculation of the ratio offered no advantage for predicting positive oral food challenge (OFC) to milk, egg, wheat or soy compared with sIgE alone. A raised total IgE did not affect the 95% PPV decision points and failed to reduce the necessity for OFC where deemed necessary [19].
The APT has been shown to improve the specificity of tests when used in conjunction with a SPT and sp IgE for the diagnosis of food allergy. Test sensitivity is, however, only marginally enhanced by the addition of APT and a negative APT does not obviate the need for an OFC when food allergy is strongly suspected. Furthermore, the APT requires specialist interpretation by a health professional trained in its use and is not generally recommended for routine diagnoses of food-induced eczema. In a recent evaluation of a large number of children with eczema, the APT failed to reduce the number of OFC tests needed when food-induced eczema was suspected [20–22]. In selected centres with specialist expertise in patch testing, the APT has been used (i) where there is a suspicion of food allergy without predictive specific IgE levels or positive SPT; (ii) in patients with severe and/or persistent eczema with unknown trigger factors; and (iii) in patients with multiple IgE sensitizations without proven clinical relevance in patients with eczema. The ATP is not currently used as a diagnostic tool within the UK for the diagnosis of food allergy, outside the research setting.
Oral Food Challenge Tests
Oral food challenge testing (OFC) remains the gold standard for diagnosing food allergy. Ideally this should be performed as a DBPCFC, with both the investigator and patient blinded to the food being tested [23,24]. In everyday clinical practice, open challenge testing is carried out more frequently, with DBPCFC being carried out in selected patients where there is still doubt about the diagnosis following a positive OFC test. The aim of OFC testing is to accurately identify causative allergens which can be eliminated from the diet and to establish foods that can safely be included in the diet, thus sparing the child from unnecessary dietary restrictions [25].
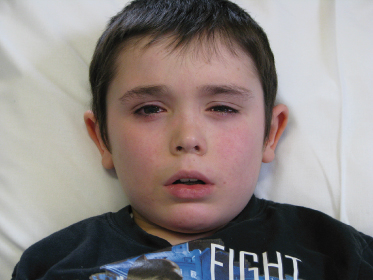
Fig. 31.2 This boy had an oral food challenge to peanut as he had never eaten peanut but had a high specific IgE to peanut. He developed facial flushing, rhinoconjunctivitis and wheezing during challenge, which responded to intramuscular (IM) adrenaline. Adrenaline is rarely required during food challenge as most reactions respond to oral and inhaled medication or need no treatment at all [26,27].
Parental and personal permission given for publication.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree