20 Evaluation and Treatment of Congenital Breast Deformities
Summary
Congenital deformities of the breast are quite commonly encountered by plastic surgeons because anatomic abnormalities associated with these conditions affect the appearance of the breasts. Each deformity or condition is unique in terms of etiology, workup, and treatment method. Each deformity can be classified under one of the subtypes of congenital deformities of the breast, including hyperplastic conditions, hypoplastic conditions, and deformational conditions. All of these conditions pose reconstructive challenges for plastic surgeons aiming to achieve symmetry and restore the natural anatomy that was hindered during the developmental process. This chapter provides a review of the most common conditions and summarizes the treatment methods for each one.
20.1 Introduction and Embryology
Congenital breast defects usually can be traced to abnormal development. Therefore, an understanding of normal breast development is critical and can help the plastic surgeon identify appropriate treatments and management of potentially associated conditions.
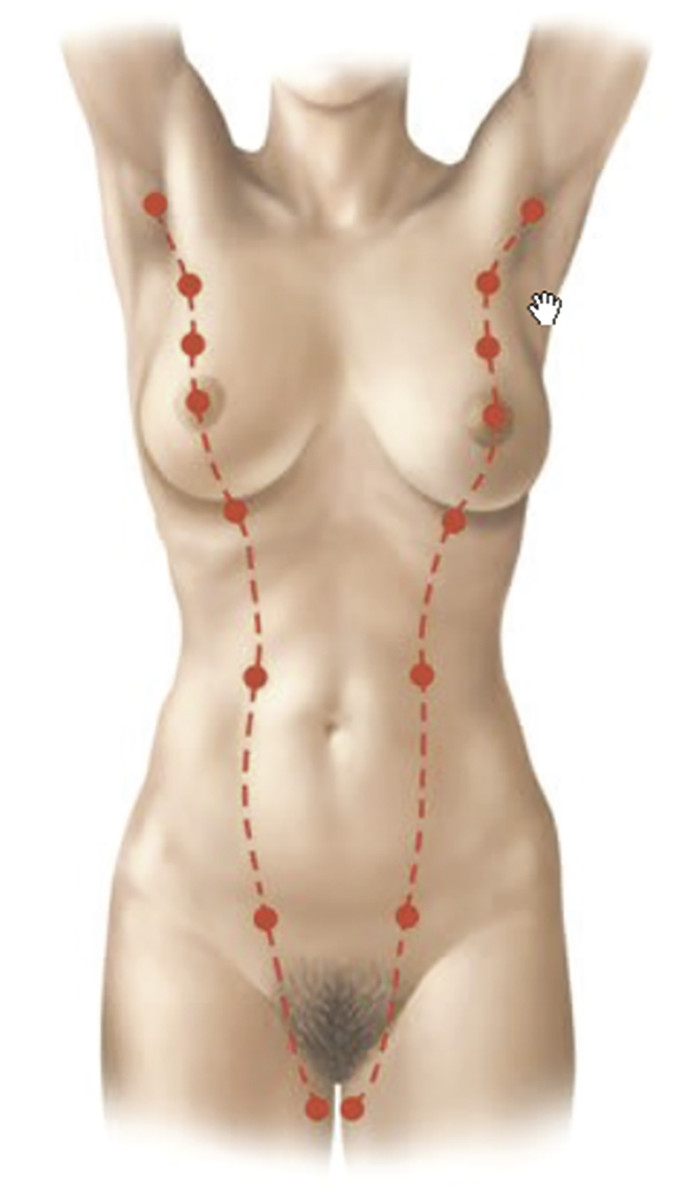
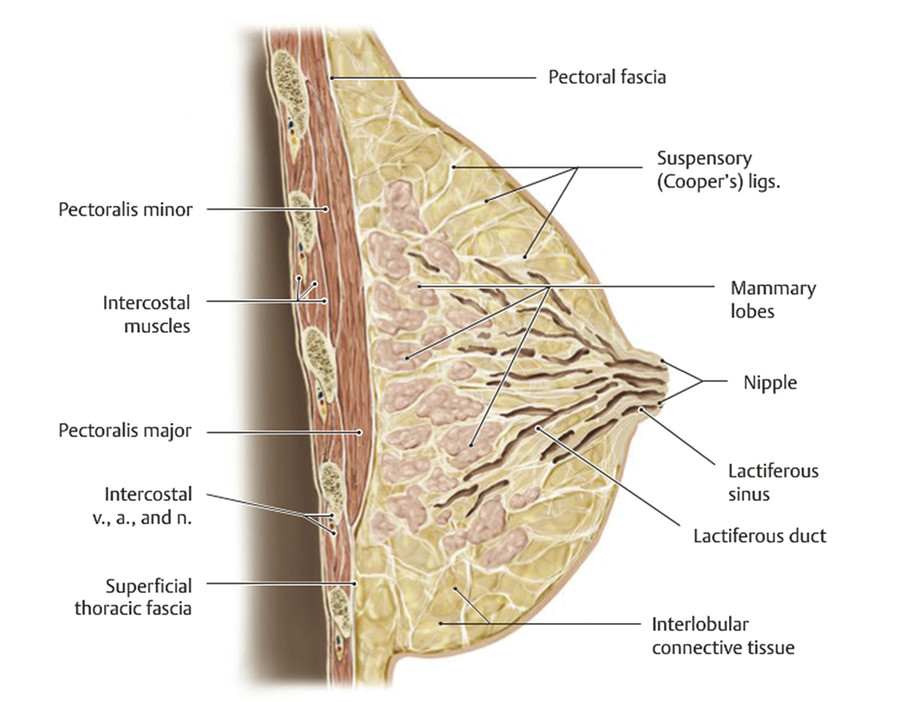
The breast is a modified sweat gland whose development begins in the fourth to sixth week of gestation. 1 , 2 Strips of thickened ectoderm appear bilaterally on the ventral surface extending from the axillae to the groin, known as mammary ridges or milk lines (Fig. 20‑1). By the seventh to eighth week of gestation, the 15–20 buds within each of the paired mammary ridges begin to involute and undergo apoptosis, with the exception of a single pair of buds (primary mammary buds) that persist at the fourth to fifth intercostal space. 1 , 2 , 3 At 8 weeks, the primary bud epithelial tissue begins to invest the underlying mesoderm, known as the disk stage. 4 The underlying mesenchymal connective tissue eventually develops into the superfascial system and Cooper’s suspensory ligaments. 3 Between 8 and 14 weeks, the epithelial tissue continues to grow deeper, completing the globular and cone stages, thereby beginning to form buds, which branch into primitive breast alveoli. 4 At 16 weeks, 15–25 epithelial branches are formed, while concurrently differentiation of sweat and sebaceous glands begins. At the start of the third trimester, lactiferous ducts and branches begin to form as small lumina develop within the mammary buds in response to placental sex hormones that initiate the canalization of the epithelial branches. 3 , 4 The canalization stage continues into childhood. The convergence of these lactiferous ducts is a shallow pit that eventually becomes the nipple. The areola begins developing at 5 months.
At birth, male and female breasts are indistinguishable, with 15–20 mammary lobules that drain into ampullae via the main lactiferous ducts. 1 After birth, the nipple starts to protrude from the areola, and further branching and terminal duct development continues until 2 years of age. Breast development is then suspended until thelarche. In North America, puberty generally starts between 8 and 12 years, during which hypothalamic release of gonadotropin-releasing hormone (GnRH) begins a hormonal cascade that resumes breast development. Increased GnRH stimulates release of the pituitary hormones follicle-stimulating hormone (FSH) and luteinizing hormone (LH), which induces maturation of ovarian follicles. These follicles release systemic progestin and estrogen, which in turn induce proliferation of mammary ductal epithelium, surrounding stroma, collecting ducts, terminal lobules, and breast buds (Fig. 20‑2). Volume increases with concomitant vascular and connective tissue proliferation. 1 Overall breast development is commonly described by the Tanner stages (Table 20‑1). 5
Tanner stage | Event |
I (preadolescent) | Papilla elevation above level of chest wall |
II (breast budding) | Breast and papilla elevation, increased areola diameter |
III | Ongoing enlargement of breasts and areolae |
IV | Elevation of areola and papilla above breast mound |
V | Elevation of papilla with regression of areola |
20.2 Hyperplastic Conditions
20.2.1 Polythelia
Polythelia, or supernumerary nipples, is the most common encountered anomaly of the pediatric breast. 4 , 6 The incidence of the condition has been reported to vary from 0.22% to 5.6%. 7 Males and females are generally affected equally, 6 , 7 and there are some reported differences in incidence between ethnic groups. 2 Cases tend to be sporadic, although reports of familial cases have been reported. 4 , 6 The etiology is failure of the mammary ridge to regress, although the cause of failure remains unknown. 4 There have been reports of associations with other ectodermal abnormalities, particularly urologic. 2 , 4 , 6
Given the pathophysiology, supernumerary nipples are found at birth anywhere along the milk line (Fig. 20‑1). However, other locations have been reported, suggesting an alternative etiology for the condition. 3 , 7 Most cases are unilateral, without right- or left-side preference, and usually occur in the area below the breast and above the lower abdominal skin. 3 , 7 Polythelia is usually recognized at birth and often initially confused with nevi or other skin lesions. The nipple can vary from having partial to complete areola, with some even with fully elevated breast tissue. 4
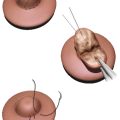
Polythelia can generally be observed. Elective treatment for irritation or cosmetic purposes can be accomplished with simple elliptical excision. Pigmented lesions are exceptions and should be treated with early prepubertal excision and histopathologic workup, as cancerous degeneration has been reported. 6 , 7 If the decision for excision is after the onset of puberty in females, glandular growth may warrant a wider excision. 6 If the supernumerary nipple to be excised is on the breast itself, magnetic resonance imaging (MRI) can confirm which nipple areolar complex (NAC) is associated with glandular and ductal tissue. Elliptical excision of the nonassociated nipple proceeds with a subsequent modified Benelli mastopexy to transpose the true NAC into the skin defect. 6 Additional workup, including physical exam, urinalysis, and renal ultrasound, should be undertaken to rule out any associated renal anomalies. 4
20.2.2 Polymastia
Polymastia, sometimes referred to as ectopic breast, is a congenital condition of extra-anatomic breast tissue. It has a lower incidence than polythelia, 7 occurring in 0.1–1.0% of all live births. 3 The etiology is similar to that of polythelia, likely due to failure of the mammary ridge to regress in utero. 4 There is a female predominance, 2 and generally cases are sporadic in nature. However, familial inheritance has been reported. Polymastia can also be associated with chromosomal abnormalities and other anomalies of the thoracic or urogenital system. 3 , 6 , 7
Unlike polythelia, polymastia often goes unnoticed until puberty, when the glandular tissue responds to hormonal influences. 4 , 6 Pregnancy and lactation involve similar hormonal changes that can also trigger latent polymastia. This condition also generally occurs along the milk line, most commonly in the axilla (Fig. 20‑1). 3 , 4 In fact, it is often mistaken for lymphadenopathy or a lipoma. The ectopic breast tissue most commonly manifests as a scanty amount of tissue with a small NAC, but presentation can vary widely. 2 , 4 Because breast tissue responds to systemic signaling, accessory breast tissue is subject to the same physiologic changes and disease processes as normal breast tissue. 2 , 4
Resection is generally considered for cosmetic reasons as well as for pathologic risk reduction. Timing of resection generally occurs after breast development has completed to avoid continued growth of retained tissue. Type of resection depends on size and location. 7 If there is a third distinct breast mount, simple mastectomy with avoidance of inframammary fold (IMF) disruption and soft tissue envelope of the remaining breast is warranted. If the ectopic tissue is adjoined to the normal breast, tissue-sparing techniques with skin de-epithelialization and accessory nipple excision can be performed. Because of high complication rates of resection in the axilla, liposuction can be useful for reduction of fatty components of the accessory tissue and definition of the planes between the accessory tissue and underlying axilla. 2 Follow-up is warranted for continued cancer surveillance of any possible residual tissue. 6 The common etiology of polythelia and polymastia brings about a wide variety of presentations. These are organized by the Kajava classification system (Table 20‑2).
20.2.3 Virginal Hypertrophy or Mammary Hyperplasia
Mammary hyperplasia or virginal hypertrophy is a rare condition of unknown etiology. 3 , 4 , 5 It is thought that the glandular tissue is hyperresponsive to normal levels of hormones, causing rapid and disproportionate breast growth. Notably, the fatty and fibrous elements of the breast tissue are increased rather than the glandular component. 3 Despite the proposed hormonal etiology, there is no association with obesity or increased risk of breast cancer. 3 , 4 This condition is typically sporadic; however, one case of hereditary pattern has been reported. 4
Mammary hyperplasia usually presents shortly after puberty, approximately between the ages of 11 and 16. If it presents before puberty, cases are usually bilateral. Virginal or postpubertal presentation can be unilateral or bilateral. 6 Patients generally have normal endocrine studies and otherwise normal growth of the remainder of the body. 6 Growth occurs rapidly and is disproportionate to the rest of the body. 4 Sternal notch–to-nipple distance can increase several centimeters per month. Onset of symptoms can occur over the course of a few months. The rapidity of expansion causes skin changes such as striae, thin skin, and dilated veins. The breast is dense and tender on physical exam. Patients usually present with significant discomfort due to the weight of the breast, and this can cause significant psychological stress.
The goals of treatment are volume reduction, symmetry, and anatomically correct NAC. 6 Timing of definitive surgery is usually delayed until the end of puberty, after breast growth has been static for approximately 1 year, to avoid revisions. 3 , 6 However, if patients are highly symptomatic, earlier surgical treatment is warranted. Standard treatment is breast reduction; however, regrowth after reduction can occur. Antiestrogenic medical adjuncts (such as medroxyprogesterone acetate, dydrogesterone, tamoxifen, and bromocriptine [if pregnant]) play a significant role in decreasing the rates of regrowth after reduction. 4 Recalcitrant regrowth may require revisional surgery (or mastectomy for extreme cases) with free nipple grafts or nipple areolar reconstruction. 4 Counseling with a psychiatrist or psychologist is recommended in the treatment plan, especially to ensure that patients can accept the risks of surgery including potential scarring, diminished nipple sensitivity, and reduced ability to breastfeed. 3
20.3 Hypoplastic Conditions
20.3.1 Asymmetry
Breast asymmetry is a generic term that refers to the variable hypoplasia of one or both breasts. It occurs frequently (as high as 80% in patients presenting to a single plastic surgery clinic desiring augmentation or reduction mammaplasty) and has a large variety of presentation on a continuum from minor to more than an entire size difference between breasts. 3 , 8
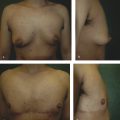
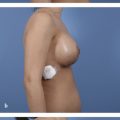
Because the goal of treatment is symmetry, treatment is optimally performed after full development to allow for planning. 2 For mild asymmetries, augmentation of the smaller breast, with or without reduction or mastopexy of the larger breast, can help achieve symmetry (Fig. 20‑3, Fig. 20‑4). For more severe asymmetries, staged reconstruction with tissue expansion and overinflation can allow for breast ptosis and a larger skin envelope before the permanent implant is inserted. 3 Lipofilling can help adjust for small asymmetries or revisions. 2 If both breasts are hypoplastic, differential augmentation of both breasts may be required. 6 It is important to note that differential physiologic changes can occur to each reconstructed breast with aging, weight gain, or pregnancy, which may require additional procedures. 3
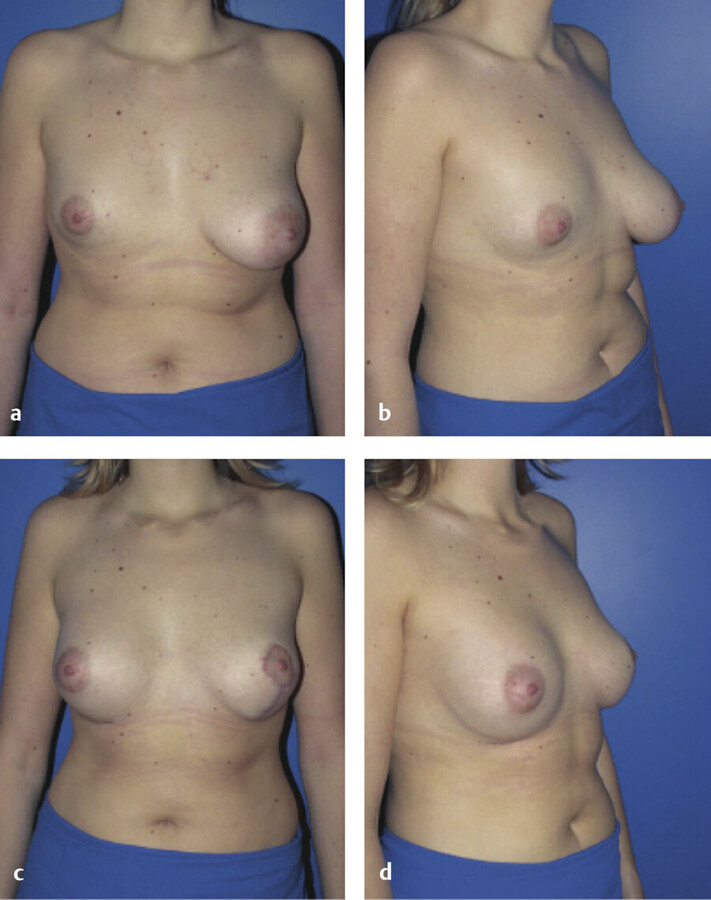
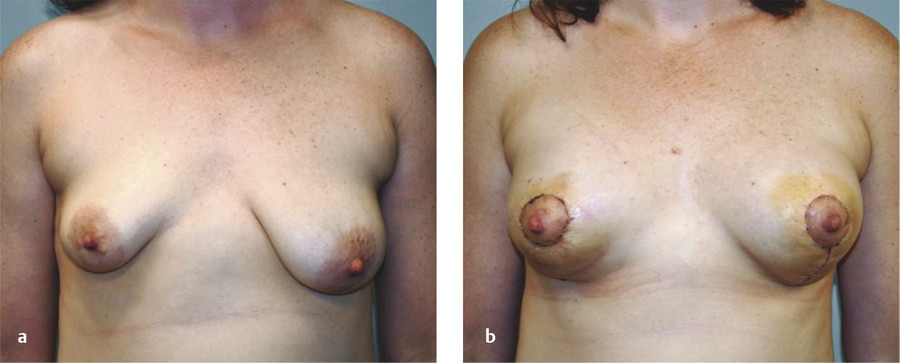
Elsahy’s classification system for asymmetry is shown in Table 20‑3. 9 The most common types of asymmetry in one study were found to be type IV (bilateral asymmetric micromastia) followed by type V (bilateral asymmetric macromastia). 8 Classification of patients to a particular asymmetry category can assist with preoperative surgical planning.
Elsahy asymmetry classification | Definition |
Type I | Unilateral micromastia |
Type II | Unilateral macromastia |
Type III | Micro- and macromastia |
Type IV | Bilateral asymmetric micromastia |
Type V | Bilateral asymmetric macromastia |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree