Lidocaine
Lidocaine is usually administered as a 0.5–2% (5–20 mg/mL) solution. The maximum recommended dose in patients 4 years of age and above is 4.5 mg/kg, which is equivalent to a maximum volume of 0.45 mL of 1% lidocaine per kilogram of body weight [4].
The addition of adrenaline counteracts the natural vasodilatory effect of lidocaine and hence decreases the rate of lidocaine absorption, increases its duration of action and reduces the risk of systemic toxicity. As an added benefit, the localized vasoconstriction induced by adrenaline also decreases the amount of intraoperative bleeding during surgical excision. We recommend a maximum adrenaline dosage equivalence of 0.01 mg/mL (concentration of 1 : 100,000). The maximum recommended dose of lidocaine combined with adrenaline is 6 mg/kg, equivalent to a maximum volume of 0.6 mL per kilogram of body weight. For an average newborn weighing 4 kg, the maximum volume of lidocaine combined with adrenaline is only 2.4 mL. It is not advisable to use adrenaline in procedures involving end-arterial structures, e.g. distal digits, penis or pinna of the ear, as there is a risk of vasoconstriction of the distal end arteries and subsequent cutaneous necrosis.
A disadvantage of lidocaine as a local anaesthetic is the pain associated with its injection. The addition of 8.4% sodium bicarbonate (1 mmol/mL) to 1% lidocaine (with or without adrenaline) in a ratio of 1 : 10 has been shown to decrease pain without significant alteration of onset, extent or duration of anaesthesia [5]. The increase in the pH of the mixture, as well as faster nerve penetration as a result of an increase in the proportion of the uncharged and more lipophilic form of the amide molecule, may explain the reduction in pain. Lidocaine is more soluble and has a longer shelf-life at acid pH. After the addition of bicarbonate as a buffer, the mixture should be refrigerated, as the initial concentration of lidocaine falls to 66% after 4 weeks at room temperature. Refrigeration at a temperature of 0–4°C maintains lidocaine at 94.5% of its initial concentration after 4 weeks [6]. The average duration of anaesthesia with plain lidocaine is 40–60 min, and is decreased to 30 min with the addition of sodium bicarbonate [7]. For extended procedures, the addition of adrenaline to lidocaine is recommended.
Adverse Effects
Local adverse effects related to the injection include pain, haematoma or ecchymosis, nerve damage and vasovagal syncope.
Ester anaesthetics are more likely than amide anaesthetics to cause allergic reactions. Allergic reactions to ester anaesthetics are related to their metabolism to para-aminobenzoic acid, a potential allergen. True allergic reactions to lidocaine and other amide anaesthetics are rare, making up less than 1% of adverse reactions [3]. Cross-reactivity of allergic reactions between the amide and ester anaesthetic classes is rare.
Injection into a highly vascularized area, accidental intravascular injection or overdosage results in high, possibly toxic, systemic concentrations which may cause adverse central nervous system (CNS) and cardiovascular effects. Initially, stimulation of the nervous system occurs, causing perioral tingling and numbness, anxiety, apprehension, restlessness, nervousness, disorientation, confusion, dizziness, blurring of vision, twitching, shivering or seizures. At greater doses, neurodepression can occur, resulting in unconsciousness, respiratory depression or coma. Cardiovascular toxicity is generally noted after CNS symptoms have developed [8]. Effects include prolonged electrocardiographic intervals, bradycardia, hypotension, decreased myocardial contractility and cardiac arrest. Bupivacaine is especially associated with cardiac toxicity: an increase in PR interval and major widening of QRS usually precede arrhythmias (ventricular tachycardia, rarely torsades de pointe).
References
1 Calverley RK, Scheller MS. Anesthesia as a specialty: past, present and future. In: Barash PG, Cullen BF, Stoelting RK, eds. Clinical Anesthesia, 2nd edn. Philadelphia: JB Lippincott, 1992:3–33.
2 Grekin RC, Auletta MJ. Local anesthesia in dermatology surgery. J Am Acad Dermatol 1988;19:599–614.
3 Norris RL. Local anesthetics. Emerg Med Clin North Am 1992;10:707–18.
4 McEvoy GK, Snow EK, Kester L et al. AHFS Drug Information 2006. Authority of the Board of American Society of Health-System Pharmacists, Bethesda, MD, 2006:3201–5, 3198–200.
5 Christoph RA, Buchanan L, Begalid K et al. Pain reduction in local anesthetic administration through pH buffering. Ann Emerg Med 1988;17:117–20.
6 Larson PO, Ragi G, Swandby M et al. Stability of buffered lidocaine and adrenaline used for local anesthesia. J Dermatol Surg Oncol 1991;17:411–14.
7 Holmes SG. Choosing a local anesthetic. Dermatol Clin 1994;12:817–23.
8 Grekin RC, Auletta MJ. Local anesthesia in dermatology surgery. J Am Acad Dermatol 1988;19:599–614.
Techniques to Decrease the Pain of Injection
There are several techniques that may be employed to decrease the pain of lidocaine infiltration [1]. Prior treatment of the injection site with topical anaesthetics such as EMLA cream or liposomal lidocaine (LMX cream), pH buffering of the anaesthetic solution, using small gauge needles (e.g. 30 gauge), warming of the anaesthetic to body temperature, cooling the injection site with ice or ethyl chloride spray, and a slow injection rate minimize the pain of the injection. Counter-stimulation techniques, such as pinching or rubbing of the injection site prior to infiltration, may reduce the pain of injection by activating substance-P fibres in the skin [2].
Topical Anaesthetics
In order to gain access to the sensory nerve endings, cutaneous analgesics must diffuse through the stratum corneum, which is the major barrier preventing local anaesthetics from penetrating the deeper tissue layers [3]. None of the topical agents to date, even when used under occlusion, produces reliable anaesthesia of the palms and soles.
Topical anaesthetics are convenient, cost-effective and associated with few adverse effects. Novel anaesthetic formulations and transdermal delivery systems are promising and may result in even more effective and safer topical analgesia in the future [4].
Eutectic Mixture of Local Anaesthetics (EMLA)
EMLA cream is a mixture of 2.5% lidocaine and 2.5% prilocaine. EMLA melts at a lower temperature than lidocaine or prilocaine alone, resulting in a stable cream at room temperature. It has been shown in numerous clinical trials to be safe and efficacious for needlestick, venepuncture, intravenous catheterization, lumbar puncture, debridement of ulcers, ablative treatment of molluscum contagiosum, laser treatment on skin and genital mucosa, and other superficial skin surgery [5]. EMLA cream alone does not appear to provide sufficient analgesia for deep biopsies or scalpel excision of skin. Standard usage requires application of the product on the skin surface with an occlusive wrap, such as Tegaderm or Cellophane wrap, for 60–120 min. Patch preparations may allow easier application with equivalent efficacy [6]. The maximum recommended doses for EMLA on intact healthy skin in children are shown in Table 190.2. The depth and degree of analgesia is related to the duration of application. The maximum depth of analgesia is 5 mm. Mucous membranes, genital skin and diseased skin with an impaired skin barrier function absorb more rapidly, allowing for shorter application times (5–40 min). The recommended application times for EMLA cream in selected procedures are shown in Table 190.3.
Table 190.2 Recommended eutectic mixture of local anaesthetics dosing on intact and healthy skin in children
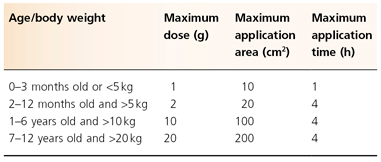
Table 190.3 Recommended application times for EMLA cream in selected procedures
Adapted from Kearns et al. 2003 [31].
| Indication | Application time (min) |
| Molluscum contagiosum | 30–60 (15 in children with atopic dermatitis) |
| Skin biopsy (pretreatment) | 60 |
| Condylomata acuminata | 5–15 |
| Port wine stain (pulsed-dye laser) | 60 |
| Leg ulcers (debridement) | 30 |
| Vaccination | 60 |
EMLA commonly causes blanching or erythema at the site of application. It may occasionally cause transient, local irritation, swelling, purpura or pruritus. Periorbital application should be avoided as corneal ulceration and irritation are possible adverse effects [7]. Methaemoglobinaemia due to the prilocaine component, a potentially life-threatening complication, has been reported, mainly in neonates and infants less than 3 months of age as their methaemoglobin reductase pathway is immature [8]. However, two reports attributed methaemoglobinaemia in a 3-year-old toddler to the excessive use of EMLA and in a 7-month-old infant to prolonged use while receiving inhaled nitric oxide [9,10]. Use of medications associated with methaemoglobinaemia (including sulphonamides, dapsone, benzocaine and chloroquine) may increase the risk of EMLA-associated methaemoglobinaemia in infants. CNS toxicity has been reported after excessive application of EMLA over an extensive area [11].
Liposomal Lidocaine
Liposomal lidocaine (LMX) is a topical anaesthetic that is encapsulated in a phospholipid-based carrier. Liposomal vehicles facilitate and improve diffusion of the local anaesthetic through the dermis. In a systematic review, topical liposomal anaesthetics were found to be effective prior to dermal instrumentation [12]. Liposome-encapsulated lidocaine is commercially available in both 4% and 5% preparations. In comparative clinical trials, LMX (applied for 30 min) and EMLA (applied for 60 min) were equally effective in reducing the pain associated with venepuncture and intravenous catheter insertion in children [13,14]. The faster onset of anaesthesia with LMX is an advantage in paediatric clinical practice. In studies carried out in adults, LMX has been shown to produce a longer duration of analgesia as the phospholipid carrier serves to maintain a longer localization of the anaesthetic [15–17]. There have been no reports of serious adverse effects with the use of LMX. However, LMX should not be applied for more than 2 h in order to avoid excessive systemic levels of lidocaine. In children weighing less then 20 kg, LMX should not be applied to a surface area greater then 100 cm2 [18]. The absence of prilocaine prevents the risk of methaemoglobinaemia.
Lidocaine Ointment and Spray
These lidocaine formulations work well as local anaesthetics when they are applied to mucosal surfaces such as those of the oropharynx, nose, vagina and cervix. However, they are not effective when applied to intact skin, as the lidocaine molecule is too big to penetrate the stratum corneum. Hence, EMLA cream has been found to be significantly more effective than 40% lidocaine ointment, even though the concentration of lidocaine in the latter formulation is many times greater [19].
Tetracaine Formulations
Tetracaine is used in multiagent formulations for the repair of dermal lacerations. The first such agent, tetracaine/adrenaline/cocaine (TAC), was introduced in 1980. In recent years, other tetracaine-containing topical anaesthetics, such as lidocaine/adrenaline/tetracaine, have replaced TAC due to the potential of cocaine to produce adverse effects [20]. Both tetracaine and liposomal tetracaine have been found to provide equivalent, if not greater, efficacy than EMLA for instrumentation of intact skin [21,22].
Lidocaine/Tetracaine Patch
A lidocaine/tetracaine patch is a topical agent that consists of a eutectic formulation of 70 mg lidocaine and 70 mg tetracaine and uses an oxygen-activated heating element to enhance delivery of the local anesthetic. The temperature of the patch increases once removed from the package, which subsequently warms the underlying skin after application. In one study it provided appropriate analgesia after 20 min of application for venepuncture in children; only transient and minor side-effects, such as erythema and oedema, were noted [23].
Subcutaneous Infusion Anaesthesia
In the tumescent technique used for subcutaneous infusion anaesthesia, large volumes of highly diluted local anaesthetics, e.g. ropivacaine or prilocaine, are instilled into the subcutaneous layer by infusion pumps [24,25]. This provides profound perioperative anaesthesia owing to slow absorption from the relatively avascular subcutaneous fat. It also provides hydrodissection of the skin from underlying vessels and nerves, facilitating surgery. However, further studies need to be done to evaluate the feasibility of this technique in paediatric dermatological surgery [26].
Iontophoresis Devices
Iontophoresis devices have been advocated for needleless delivery of local anaesthetics. A low-voltage direct current is applied to skin immersed in a local anaesthetic solution or placed under an anaesthetic-impregnated patch, facilitating transfer across the stratum corneum. Lidocaine iontophoresis has a rapid onset of anaesthesia (within 10 min) and appears to be as efficacious as EMLA cream and local lidocaine injection in providing pain relief for intravenous cannulation in children [27–30]. A study conducted in children 5–15 years of age found that the subjects tolerated iontophoresis well and systemic levels of lignocaine were low [31].
A prospective trial, evaluating lignocaine iontophoresis in 60 children undergoing shave biopsy, curettage, injection and punch biopsy, revealed that most of the subjects did not require any supplemental anaesthesia. No significant adverse events were reported [32].
Needle-Free Injection Devices
Needle-free injection devices use high gas pressure to accelerate fine drug particles to supersonic speed and deliver them into the skin. Several clinical trials studying paediatric patients demonstrated the clinical efficacy of needle-free injections [33–35]. However, another study showed that a needle-free injection device is not completely painless or cost-effective [36].
Topical Anaesthesia for Mucosal Surfaces
Topical Cetacaine (benzocaine 14%, tetracaine 2%), benzocaine, viscous lidocaine, liposomal lidocaine and EMLA are effective topical agents for inducing mucosal anaesthesia. Anaesthetic effect is almost immediate upon application. These agents are useful for decreasing the pain of intralesional lidocaine injection, but are insufficient for scalpel surgery when used alone. In infants, the use of Cetacaine and benzocaine should be avoided because of the risk of methaemoglobinaemia [37]. While benzocaine is a known sensitizer, allergic contact dermatitis seldom occurs when it is used preoperatively, presumably because of lack of prior exposure and sensitization.
References
1 Eichenfield LF, Weilepp A. Pain control in pediatric procedures. Curr Opin Dermatol 1997;4:151–61.
2 Barnhill BJ, Holbert MD, Jackson NM, Erickson RS. Using pressure to decrease the pain of intramuscular injections. J Pain Symptom Manage 1996;12:52–8.
3 Friedman PM, Mafong EA, Friedman ES, Geronmus RG. Topical anesthetics update: EMLA and beyond. Dermatol Surg 2001;27:1019–26.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








