Inherited Localized Disorder
There is no recognized disorder in this category.
Acquired Localized Disorders
Primary localized lipoatrophies correspond to a heterogenic group of disorders whose denomination mainly depicts their clinical appearance. Insulin and centrifugal lipoatrophy can present in childhood. Annular lipoatrophy, semi-circular lipoatrophy and lipoatrophy of the ankles are clinically distinct entities mainly seen in adult women and, therefore, will only be mentioned briefly.
Centrifugal Lipoatrophy
Syn.
lipodystrophia centrifugalis abdominalis infantilis, centrifugal lipoatrophy
Definition.
Described in 1971 by Imamura and colleagues [1] under the name ‘lipodystrophia centrifugalis abdominalis infantilis’, this form of lipoatrophy is characterized by (a) a localized loss of subcutaneous fat involving the greater part of the abdomen, (b) an onset before 3 years of age, (c) a centrifugal enlargement of the depressed area, (d) slightly reddish and scaly changes in the surrounding area, and (e) no other significant abnormalities. More than 100 cases have been reported to date, mainly among Japanese [2]. Occurrence in Caucasian children appears exceptional [3].
Aetiology.
The origin of centrifugal lipoatrophy is uncertain. Although small inflammatory findings such as lymph node enlargement and peripheral inflammatory cellular infiltrate are initially present in about two-thirds of cases, systemic signs of inflammation are usually absent [2]. These findings and the fact that corticosteroids do not stop the progressive enlargement of centrifugal lipoatrophy argue against a primary inflammatory process as in other types of panniculitis. Speculation has covered several possible mechanisms: (a) a primary loss of subcutaneous fat with reactive inflammatory infiltrate and lymphadenopathy, (b) localized trauma such as friction, contusion, inguinal hernia or congenital dislocation of the hip, which have all been reported as possible triggers in some patients, and (c) intercurrent infections. The higher expression of the disorder in Japanese children, together with the description of affected dizygotic twins and siblings [4], may suggest a genetic predisposition.
Histology.
Lesions are characterized by a decrease or loss of subcutaneous fat, with the presence of inflammatory cells that are more prominent in the surrounding area. The inflammatory cell infiltrate may involve the dermis as well as the subcutaneous tissue and consists of lymphocytes, histiocytes and few plasma cells in most cases [2,3,5]. Multinucleated giant cells and foamy cells have been reported [5]. Mild vascular changes (endothelial swelling) can occur, but not apparent vasculitis.
Clinical Features.
With a 2 : 1 female : male ratio, 90% of cases are characterized by an onset before 8 years of age and an abdominal location, most often the groin or surrounding area. The initial presentation includes erythematous, bluish macules or ecchymoses with regional lymph node enlargement in about one-half of the cases. In the other half, the parents first notice the lesion only by a well-defined depression or atrophy of the skin. The lesion then spreads centrifugally to leave a central part of lipoatrophy, where subcutaneous veins become easily visible. A few patients have developed two or three lesions. In a follow-up review of cases, it was found that cessation of enlargement occurs within 3 years in 50% of patients and within 8 years in 90%, followed by spontaneous resolution or marked improvement in a majority of cases [2,5–7].
The clinical course appears rather uniform in most cases. However, a few variations were recently reported. These include extra-abdominal locations, such as the head [5,8], neck [7] and lumbar region [3,6] and non-regressing cases [9]. Adult cases are extremely rare [10–12], and whether these should be classified as large unusual semi-circular lipoatrophies is disputable.
Treatment and Prognosis.
Topical and oral corticosteroids have been used with little benefit. They are usually effective at decreasing the peripheral inflammation/erythema but do not halt the progressive centrifugal extension [2]. Although most cases spontaneously stop progressing before the age of 13 years and then regress, persistence into adulthood of a lesion further complicated by angioblastoma has been reported [9].
Annular Lipoatrophy
This entity is characterized by a circular depressed band, 1 cm wide and 0.5–2 cm deep, that encircles an upper limb, usually in women aged 40–70 years. The atrophic lesion is preceded by tenderness and swelling of the entire limb. Unexplained neuralgia and arthralgic pain with muscle weakness or myopathy are frequent. Annular lipoatrophy does not spontaneously regress and may last up to 20 years. Histological findings may be minimal or show polyarteritis and strands of connective tissue replacing the subcutaneous fat. The prevalence of ‘rheumatic’ pain and associated findings suggests an underlying connective tissue disease [13–15].
Atrophy of the Ankles
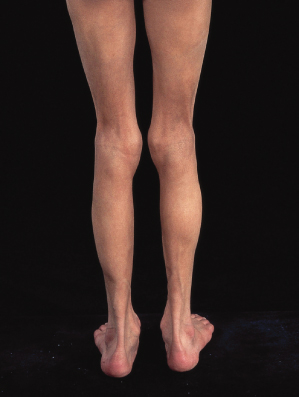
This is an extremely rare disorder, mainly characterized by its peculiar location. Less than 10 cases have been described with bilateral circumferential, asymptomatic, lipoatrophic bands, 9–11 cm wide, on the ankles. Local symptomatology and muscle involvement are absent. The disorder should be differentiated from acral lipoatrophy, which may develop as an autoimmune process (Fig. 141.2) [16–19].
Fig. 141.2 Diffuse, predominantly acral lipoatrophy in a girl with autoimmune hepatitis, alopecia and positive anti-liver and anti-kidney microsome antibodies.
Semi-Circular Lipoatrophy
Semi-circular lipoatrophy occurs more frequently than annular lipoatrophy and atrophy of the ankles, and mainly in adults. Patients present with semi-annular cutaneous depressions symmetrically distributed on the anterolateral aspects of both thighs. The lesions are asymptomatic and flesh coloured. Spontaneous resolution usually occurs within 3 years of onset. Although the aetiology of the disorder may be heterogeneous, most authors believe that the lipoatrophy follows a panniculitis of traumatic origin. Histological changes include fat atrophy replaced by collagen and mild perivascular cellular infiltrate in the dermis [20–31].
Naevoid Disorders
Localized lipoatrophy can occasionally be associated with naevoid disorders such as Becker’s naevus [32,33] or naevoid hypertrichosis [34].
Insulin Lipoatrophy
Aetiology.
Lipoatrophy following subcutaneous insulin injections was probably one of the most common causes of localized fat atrophy when diabetic patients used conventional bovine–porcine insulin preparations. As lipoatrophy was more commonly seen with longer acting insulins rather than soluble ones, and as its occurrence was greatly reduced with the availability in the early 1980s of highly purified porcine insulins [35,36], it is considered to be an immunological reaction to impurities in the insulin preparations and/or to the xenogenic insulin [37–39].
These immunological reactions should be differentiated from allergic reactions to the content of long-acting insulins, which result in generalized urticaria and not lipoatrophy [40,41].
Histopathology.
Skin biopsies show a loss of fat tissue. An increase in insulin-binding capacity is found on the edge of lipoatrophic lesions. Inflammatory changes are characteristically scant but immunofluorescence may show deposition of immunoglobulin M (IgM), C3 in the dermis and C3 in dermal blood vessels [38]. Accumulation of tryptase-positive, chymase-positive degranulated mast cells has been described with human insulin [42].
Clinical Features.
Insulin atrophy was previously seen more frequently than insulin hypertrophy, but not now. In a series of 281 patients treated with purified insulins, the prevalence of lipohypertrophy was 27% and lipoatrophy 2.5% [35]. It is a cosmetically distressing complication, which presents as a non-inflammatory, painless, small to large dimple at insulin injection sites. It usually develops within 3 years of starting insulin and is more common in children and women. Most cases are associated with higher levels of insulin requirements, as insulin absorption can be delayed or variable due to the formation of avascular, fibrous scar tissue. Lipoatrophic lesions distant from the sites of injection may occur, as well as the co-existence of both fat atrophy and hypertrophy [43].
Treatment.
The use of highly purified porcine insulins with a reinforcement of careful rotational routine of injection sites resulted in a marked decrease, but not disappearance, of insulin lipoatrophy. The use of human insulin preparations, injected directly into the lipoatrophic area, is usually curative [43,44]. This often results in both a regression of the localized lipoatrophy and a reduction in insulin requirements. However, cautious optimism should prevail as lipoatrophy may occasionally complicate human insulin injections [45]. In such cases, topical cromolyn therapy may reverse early and prevent new lipoatrophic lesions [42].
References
1 Imamura S, Yamada M, Ikeda T. Lipodystrophia centrifugalis abdominalis infantilis. Arch Dermatol 1971;104:291–8.
2 Imamura S, Yamada M, Yamamoto K. Lipodystrophia centrifugalis abdominalis infantilis. J Am Acad Dermatol 1984;11:203–9.
3 Zachary CB, Wells RS. Centrifugal lipodystrophy. Br J Dermatol 1984;110:107–10.
4 Mizoguchi M, Nanko S. Lipodystrophia centrifugalis abdominalis infantilis in dizygotic twins. J Dermatol 1982;9:139–43.
5 Hagari Y, Sasaoka R, Nishiura S et al. Centrifugal lipodystrophy of the face mimicking progressive lipodystrophy. Br J Dermatol 1992;127:407–10.
6 Caputo R. Lipodystrophia centrifugalis sacralis infantilis. Acta Dermatol Venereol 1989;69:442–3.
7 Higuchi T, Yamakage A, Tamura T et al. Lipodystrophia centrifugalis abdominalis infantilis occurring in the neck. Dermatology 1994;188:142–4.
8 Hagari Y, Ikehara A, Nuno K et al. Centrifugal lipodystrophy presenting with serpiginous erytheme and alopecia. Cutis 2002;69:281–3.
9 Hiraiwa A, Takai K, Fukui Y et al. Non-regressing lipodystrophia centrifugalis abdominalis with angioblastoma (Nakagawa). Arch Dermatol 1990;126:206–9.
10 Rowland Payne CME, Harper JI, Farthing CE et al. Lypodystrophia centrifugalis. Br J Dermatol 1985;113(Suppl):100–1.
11 Franks A, Verbov JL. Unilateral localized idiopathic lipoatrophy. Clin Exp Dermatol 1993;18:468–9.
12 Vieira Serrão V, Barata Feio A, Localized abdominal idiopathic lipodystrophy. Dermatol Online J 2008;14:15–20.
13 Bruinsma W. Lipoatrophia annularis, an abnormal vulnerability of the fatty tissue. Dermatologica 1967;134:107–12.
14 Rongioletti F, Rebora A. Annular and semicircular lipoatrophies. J Am Acad Dermatol 1989;20:433–6.
15 Ferreira-Marques J. Lipoatrophia annularis. Ein Fall einer bisher nicht beschriebenen Krankheit der Haut. Arch Dermatol Syph (Berlin) 1953;195:479–91.
16 Jablonska S, Szczepanski A, Gorkiewicz A. Lipoatrophy of the ankles and its relation to other lipoatrophies. Acta Dermatol Venereol 1975;55:135–40.
17 Roth DE, Schikler KN, Callen JP. Annular atrophic connective tissue panniculitis of the ankles. J Am Acad Dermatol 1989;21:1152–6.
18 Shelley WB, Izumi AK. Annular atrophy of the ankles. Arch Dermatol 1970;102:326–9.
19 Dimson OG, Esterly NB. Annular lipoatrophy of the ankles. J Am Acad Dermatol 2006;54: S40–S42.
20 Baurle G, Hanke E. Lipodystrophia semicircularis: ein rein kosmetisches Problem? Artzl Kosmetol 1983;13:135–41.
21 Bloch PH, Runne U. Lipotrophia semicircularis beim Mann. Hautarzt 1978;29:270–2.
22 Karkavitsas C, Miller JA, Kirby JD. Semicircular lipoatrophy. Br J Dermatol 1981;105:591–3.
23 Hodak E, David M, Sandbank M. Semicircular lipoatrophy: a pressure-induced lipoatrophy? Clin Exp Dermatol 1990;15:464–5.
24 Ayala F, Lembo G, Ruggiero F et al. Lipoatrophia semicircularis. Dermatologica 1985;170:101–3.
25 Thiele B, Ippen H. Multilokulare progrediente Lipatrophia semicircularis. Hautarzt 1983;34:292.
26 Mascaro JM, Ferrando J. Lipoatrophia semicircularis: the perils of wearing jeans? Int J Dermatol 1982;21:138–9.
27 Leonforte JF. Lipoatrophia semicircularis associated with an osseous cyst. Cutis 1983;31:428.
28 Gshwandtner WR, Munzberger H. Lipoatrophia semicircularis. Wien Klin Wochenschr 1975;87:164–8.
29 Peters MS, Winkelmann RK. The histopathology of localized lipoatrophy. Br J Dermatol 1986;114:27–36.
30 Nagore, E, Sanchez-Motilla JM, Rodriguez-Serna M et al. Lipoatrophia semicircularis – a traumatic panniculitis: report of seven cases and review of the literature. J Am Acad Dermatol 1998;39:879–81.
31 Gruber PC, Fuller LC. Lipoatrophy semicircularis induced by trauma. Clin Exp Dermatol 2001;26:269–71.
32 Van Gerwen HJ, Koopman RJ, Steijlen PM et al. Becker’s naevus with localized lipoatrophy and ipsilateral breast hypoplasia. Br J Dermatol 1993;129:213.
33 Cox NH. Becker’s naevus of the thigh with lipoatrophy: report of two cases. Clin Exp Dermatol 2002;27:27–8.
34 Cox NH, McClure JP, Hardie RA. Naevoid hypertrichosis: report of a patient with multiple lesions. Clin Exp Dermatol 1989;14:62–4.
35 McNally PG, Jowett NI, Kurinczuk JJ et al. Lipohypertrophy and lipoatrophy complicating treatment with highly purified bovine and porcine insulins. Postgrad Med J 1988;64:850–3.
36 Young RJ, Steel JM, Frier BM et al. Insulin injection sites in diabetes: a neglected area? BMJ 1981;283:349.
37 Kahn CR, Rosenthal AS. Immunologic reaction to insulin: insulin allergy, insulin resistance, and the autoimmune insulin syndrome. Diabetes Care 1979;2:283–95.
38 Reeves WG, Allen BR, Tattersall RB. Insulin-induced lipoatrophy: evidence for an immune pathogenesis. BMJ 1980;280:1500–3.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








