An estimation of the relevant risk associated with anti-Ro/SSA positivity and the NLE syndrome is helpful for counselling women and potential mothers. Approximately 2–4% of anti-Ro/SSA positive pregnancies will result in an NLE-affected baby; however, if the woman has had a previously affected infant this risk rises to 20% [7,11,12].
Aetiology and Pathogenesis.
Neonatal lupus erythematosus is a passively acquired autoimmune disease, related to anti-Ro/SSA and, in some cases, anti-La/SSB. Rare cases of NLE due to anti-RNP without detectable Ro/SSA and La/SSB have also been reported [13–15] but anti-RNP antibodies as a cause of NLE have been associated only with neonatal skin disease in the reported literature to date.
Ro, La and RNP Ribonucleoproteins
Ro (also termed SSA, Sjögren syndrome antigen A) is a ribonucleoprotein encoded on chromosome 1q. It gives a discrete nuclear speckled pattern on immunofluorescence [16] but it is also present within the cytoplasm and its expression on keratinocyte cell membranes may be induced by ultraviolet light [17]. The precise cellular function of Ro is unclear; it is a highly conserved protein which in higher eukaryote cells binds to small cytoplasmic RNAs [18]. Ro appears to play a role in small RNA quality control, enhancement of cell survival following ultraviolet exposure [18] and sequestration of defective ribonuclear proteins to protect against autoantibody development [19]. Interestingly, removal of the Ro protein ortholog (Rsr) from the extremely radio-resistant bacterium Deinococcus radiodurans renders the organism more susceptible to UV damage [20] and mice lacking the Ro protein display increased sensitivity to UV irradiation as well as an autoimmune syndrome with antiribosome antibodies and glomerulonephritis [19].
La (also termed SSB, Sjögren syndrome antigen B) is another ribonucleoprotein, encoded on chromosome 2. The La protein plays a role in RNA polymerase III synthesis and transport [21]. The antigen for RNP (ribonuclear protein) is a small nuclear RNA–protein complex, U1.
Disease Associations
The presence of anti-Ro/SSA antibodies in adulthood may be associated with primary or secondary Sjögren syndrome, SLE, subacute cutaneous lupus, rheumatoid arthritis and primary biliary cirrhosis. Anti-La/SSB antibodies are associated with primary and secondary Sjögren syndrome and SLE; they are rarely seen in the absence of anti-Ro/SSA antibodies. Anti-RNP antibodies in adulthood are associated with a broad range of autoimmune diseases, including SLE and mixed connective tissue disease. Autoantibody positivity may precede disease manifestations by many years; hence mothers of babies with NLE may be asymptomatic.
Maternofetal Transfer of Autoantibodies
Maternal anti-Ro/SSA and anti-La/SSB IgG autoantibodies bind to the placental neonatal Fc receptor, allowing transplacental passage and resulting in fetal tissue injury [22]. Maternal blood contains IgM, IgA and IgG autoantibodies, whereas only IgG autoantibodies cross the placenta and hence are present in infants, consistent with selective transplacental transfer [23]. Breast milk is a potential source of autoantibodies, but breastfeeding was not a significant risk factor for NLE in a prospective series of 32 children born to Ro-positive mothers, supporting the role of placental maternofetal transfer as the major source of autoantibodies in NLE pathogenesis [24].
Disease in the offspring correlates with the presence of maternal antibodies in the fetal and neonatal circulation and disappears, with the exception of cardiac injury, with the clearance of maternal antibodies by 8–12 months of age [11]. Interestingly, neither the maternal titre of anti-Ro/SSA antibody nor the presence/absence of active SLE in the mother predicts the development of NLE in the offspring, although high-titre anti-Ro/SSA antibodies are frequently seen in affected cases [11].
Pathogenesis of NLE Manifestations
It is likely that not all anti-Ro/SSA antibodies are pathogenic, in view of the fact that only approximately 2% of babies born to anti-Ro/SSA positive mothers develop NLE [7]. Epitope mapping and epidemiological studies have investigated the predictive value of different anti-Ro subtypes, including Ro 52 and Ro 60, but results are inconclusive [11]. Skin-specific epitopes to which the Ro and La autoantibodies react have not been identified to date. IgG crosses the placenta in increasing amounts in the second trimester, coinciding with the time period in which congenital heart block develops. Diffuse cardiomyopathy may develop but it appears that the extremely vascular atrioventricular node is at particular risk of tissue injury. In severe cases of diffuse cardiomyopathy, calcification and endomyocardial fibrosis are described. Postmortem studies demonstrate immunoglobulin and complement deposits, mononuclear cell infiltration and fibrosis of the fetal heart. Animal studies have demonstrated that anti-Ro/SSA and anti-La/SSB antibody fractions from human sera can induce cardiac conduction defects in rabbit heart tissue analogous to NLE [25], but the precise roles of anti-Ro/SSA and anti-La/SSB antibodies in the clinical pathological process remain unclear [26].
Heterogeneity Due to Genetic and Environmental Modifiers in Mother and Infant
Anti-Ro/SSA with or without anti-La/SSB antibodies are therefore necessary but not sufficient for the development of fetal disease [11] and their effects within the syndrome of NLE are heterogeneous. The relatively low incidence of NLE in babies born to mothers with anti-Ro/SSA illustrates that there must be other significant aetiological factors (both maternal and fetal) contributing to tissue injury in NLE; these are currently ill-defined but twin studies suggest that they are likely to be both genetic and environmental [11], as illustrated by discordance in twin [27] and triplet pregnancies [28,29] and the incomplete risk in subsequent pregnancies.
Ultraviolet light exposure may precipitate and/or exacerbate the cutaneous manifestations of NLE, but the presence of lesions at birth indicates that ultraviolet light is not essential for disease expression.
Anti-Ro/SSA positive women who are also HLA-DR3 are more likely to have high-titre anti-Ro/SSA antibodies [30]. Anti-5-hydroxytryptamine (HT)4 antibodies have been proposed as an additional risk factor contributing to cardiac disease in NLE, but only in a small subset of infants [31]. Hence at present the genetic and environmental factors determining susceptibility to NLE remain ill defined and the search is ongoing for a reliable biomarker to indentify infants at greatest risk of NLE, particularly congenital heart block.
Clinical Features.
The clinical features of NLE include cutaneous, cardiac, haematological, neurological and hepatic signs and symptoms; these features, their relative prevalence and duration are summarized in Table 14.2.
Table 14.2 Clinical features of neonatal lupus erythematosus
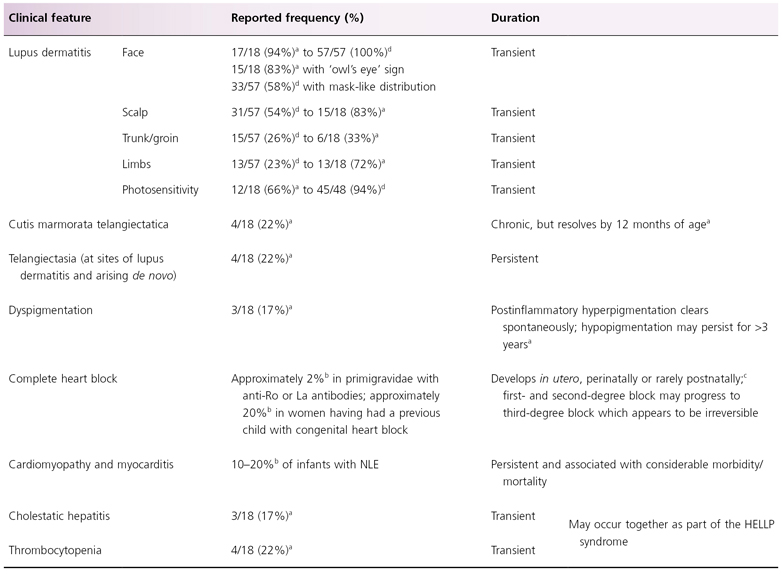
a Based on a retrospective series of 18 cases of anti-Ro positive cutaneous NLE reported by Weston et al. 1999 [23].
b Based on a review of published articles from 1977 to 2009 [11].
c Single case report [59].
d Based on a series of 57 children with cutaneous NLE but no heart block, as part of the Research Registry for Neonatal Lupus [9,15].
HELLP, Haemolysis, Elevated Liver enzymes and Low Platelets.
Cutaneous Manifestations
Cutaneous features represent the most common manifestation of the NLE syndrome and may be the only manifestation in a proportion of cases: 4/10 in one recent series [32] and 44/57 in a series of babies in the Research Registry for Neonatal Lupus [15].
Cutaneous NLE is characterized by annular, erythematosus, macular or oedematous lesions, with or without fine scaling (Figs 14.1–14.3). Neonatal lupus does not usually show the follicular plugging and dermal atrophy which is characteristic of discoid or chronic cutaneous lupus erythematosus but its annular and polycyclic lesions may resemble subacute cutaneous lupus erythematosus in adults [33] both clinically and histologically [23].
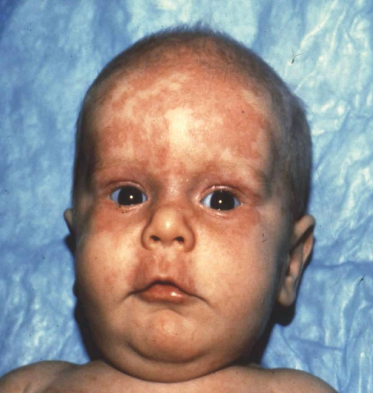
Fig. 14.1 Annular rash on face. A 2-month-old anti-Ro/SSA positive male infant (younger sibling of the child in Fig. 14.6), showing characteristic erythematosus annular lesions on the forehead and periorbital skin.
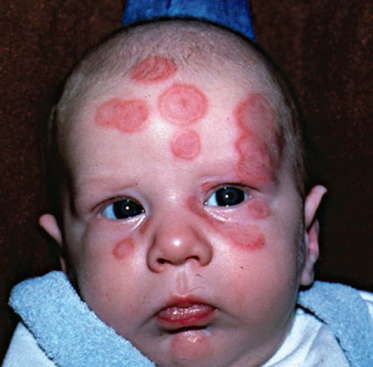
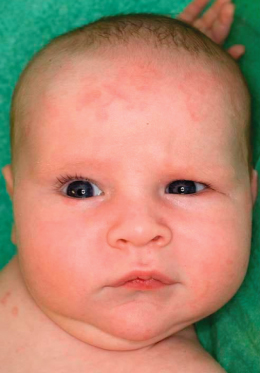
Fig. 14.2 Annular rash on abdomen. This baby boy developed annular lesions on his back and scalp, followed by abdominal and facial skin, from 3 weeks of age; he also showed livedo reticularis on the limbs and trunk and had elevated liver enzymes (AST 108 U/L, ALT 78U/L), but no other manifestations of NLE. The annular erythematosus lesions resolved by 6 months of age, with the exception of some residual telangiectasiae on eyelids and frontal scalp. The child’s older sister had a history of NLE (shown in Fig. 14.5); his mother had Sjögren’s syndrome and photosensitivity; the maternal grandmother had discoid lupus erythematosus.
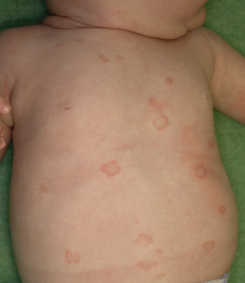
Fig. 14.3 Erythematous and scaling plaques on the arm of an infant with NLE.
Courtesy of Adelaide Herbert.
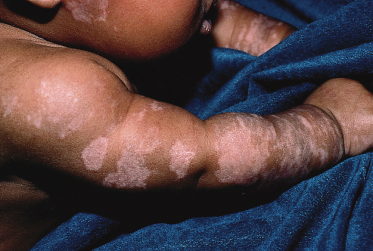
Neonatal lupus lesions do not characteristically result in dermal scarring, although hypopigmentation, epidermal atrophy, telangiectasia (Fig. 14.4) and scarring (Fig. 14.5a) have all been reported [4,34,35]. Livedo reticularis and cutis marmorata changes may be a feature [36] but this appearance is common in the neonatal period and not specific for NLE. Papulosquamous and crusted lesions [23] (Fig. 14.5b) have also been reported, as well as isolated reports of morphoea and panniculitis. Petechiae may occur as a result of thrombocytopenia (see below).
Fig. 14.4 Telangiectasia on scalp in association with NLE. Telangiectatic lesions are a manifestation of NLE, as shown here on the scalp skin of the same child as seen in Fig. 14.5. Telangiectasia may persist for longer than the classic annular erythematous lesions of NLE.
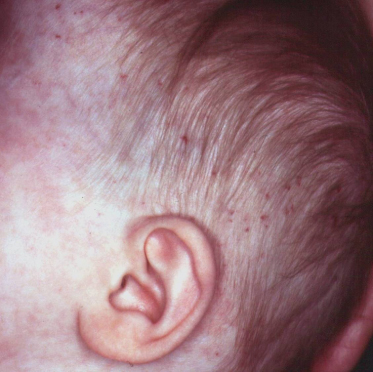
Fig. 14.5 This 8-year-old girl (a sister of the child in Fig. 14.2) had neonatal lupus, presenting at 3 weeks of age with inflammatory, crusted annular lesions on the trunk and scalp (a) as well as thrombocytopaenia and transiently elevated liver transaminases. The inflammatory lesions resolved by 5 months of age, with residual atrophic scarring on the abdomen, a rare complication of NLE (b).
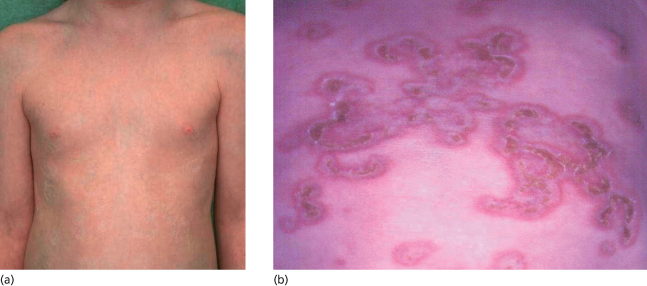
Cutaneous manifestations show a predilection for the face and scalp, particularly the periorbital and malar regions, resulting in the so-called ‘owl’s eye’ or ‘racoon’ sign (periorbital erythema) seen in approximately 80% of cutaneous NLE cases [23] (Fig. 14.6). Skin manifestations may be precipitated or exacerbated by ultraviolet light exposure [37–39], including phototherapy for hyperbilirubinaemia [40], which may in part explain the scalp and facial predilection. However, sites not exposed to the sun may also be affected, including the trunk and limbs and rarely the vulva, buttocks, palms and soles.
Fig. 14.6 Annular rash in periorbital and temporal distribution producing the ‘owl’s eye’ appearance. A 9-week-old anti-Ro/SSA and anti-La/SSB positive male infant (an older sibling of the infant in Fig. 14.1), showing characteristic ‘owl’s eye’ appearance. He was otherwise well and the cutaneous lesions resolved at 12 weeks of age. His mother, though also Ro, La and ANA positive (1:640), was asymptomatic at the time of delivery.
Skin lesions may be mild (Fig. 14.7) and transient and therefore unrecognized, so that their prevalence and natural history are difficult to define. The mean age for development of cutaneous NLE is 6–7 weeks but lesions may be visible at birth and onset may be delayed up to 5 months after delivery [41,42]. The majority of skin lesions disappear spontaneously by the age of 12 months, with most clearing within 6–8 months. Residual telangiectasia and postinflammatory dyspigmentation are the most chronic lesions, persisting for months or years [23].
Fig. 14.7 Subtle rash on scalp forehead in a neonate with NLE. The cutaneous manifestations of NLE may be subtle, as illustrated by this child showing cutaneous NLE lesions on the forehead and right upper trunk (the same infant is also shown in Fig. 14.2).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








