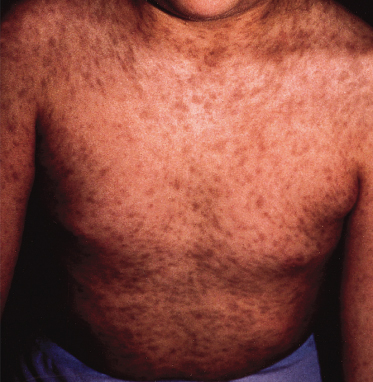
Fig. 104.2 Numerous oval-shaped grey-brown macules on the trunk of a child with erythema dyschromicum perstans.
Courtesy of the University of Southern California residents’ slide collection.
In addition to the above processes, trauma such as friction or burns may result in hyperpigmentation.
Treatment.
Initial treatment is to prevent further inflammation and depends on the underlying process. Sun protection may help to speed recovery. Treatment of the pigmentation itself is often disappointing. Topical retinoids may be used or hydroquinone, but care must be taken to protect surrounding normal skin and to avoid overtreatment resulting in hypopigmentation. Laser treatment, for example with Q-switched ruby or Q-switched Nd:YAG, may result in hyperpigmentation and reports of benefit are variable [2,3].
Drug-Induced Circumscribed Hyperpigmentation
Drugs may affect the colour of the skin by altering melanin synthesis, through deposition of a drug-related material or as a result of postinflammatory changes. A wide and expanding range of drugs are implicated. The most commonly associated are minocycline, antimalarials, oral contraceptives, cytotoxic drugs and heavy metals.
Tetracyclines (except doxycycline) may potentially all cause hyperpigmentation [4]. Tetracycline is rarely associated with blue-coloured osteomas [5]; however, most reports of hyperpigmentation are secondary to minocycline. Pigmentation with minocycline is thought to be secondary to deposition of a degradation product chelated to iron [6]. Because minocycline is a highly lipophillic drug it has excellent tissue penetration, which may contribute to deposition in various tissues. Table 104.3 summarizes the types of cutaneous pigmentation associated with minocycline [7–10].
Table 104.3 Types of cutaneous pigmentation associated with minocycline
| Pattern | Main clinical features |
| Type I | Blue-black macules at sites of scarring or inflammation, primarily acne scars |
| Type II | Blue-black, brown or slate-grey pigmentation on healthy skin, predominantly ankles, shins and arms. May be diffuse or circumscribed |
| Type III | Muddy-brown on healthy skin, generalized, symmetrical and accentuated at photoexposed sites |
Pigmentation has been reported at many sites outwith the skin, such as heart valves, sclerae, teeth, nails, mucous membranes, thyroid and breastmilk [4,10,11,12]. Pigmentation in the skin usually resolves after months or years but may be permanent.
Chloroquine and hydroxychloroquine used in the treatment of malaria or connective tissue disease may result in brownish or blue-black discoloration of the face, shins or hard palate. This is usually associated with long-term use and resolves upon cessation [13].
Bleomycin, an antibiotic used in the treatment of malignancy such as Hodgkin’s lymphoma, may produce diffuse or patchy pigmentation, which is often worse on extensor surfaces [14,15]. It may also produce a characteristic ‘flagellate dermatitis’ resulting in linear hyperpigmentation [16,17]. It has been postulated that secretion of chemotherapy agents onto the skin in sweat may explain hyperpigmentation at sites of tapes and ECG pads [18]. A number of other chemotherapy agents are associated with localized cutaneous hyperpigmentation including carmustine, 5-fluorouracil and thiotepa [19].
Heavy metals may affect pigmentation via systemic absorption or local contact. Argyria is a bluish-grey discoloration that may be localized or widespread and is due to the deposition of silver in the skin. It may be caused by silver-containing medicines or by topical silver sulfadiazine [20]. Chrysiasis is a permanent bluish-grey discoloration caused by gold salts, such as intramuscular gold used in rheumatic diseases, and is limited to sites of sun exposure [21]. Arsenic is a carcinogen and a major pollutant in drinking water in many parts of the world. It may result in patchy hyperpigmentation or hypopigmentation [22].
Pigmentation Due to Mastocytosis
In children cutaneous mastocytosis usually presents as either urticaria pigmentosa, solitary cutaneous mastocytoma or less commonly diffuse cutaneous mastocytosis. The prognosis is generally better in childhood mastocytosis than in adult disease. The cause of pigmentation in these conditions is not yet fully understood. In adult forms mutations in c-KIT have been isolated and are believed to be the cause of mast cell proliferation [23,24]. In paediatric cases evidence for c-KIT mutation is less clear although recent evidence suggests that c-KIT mutations may be more prevalent in childhood mastocytosis than previously thought [25]. c-KIT has an important role in melanocyte physiology, and loss-of-function mutations lead to depigmentation as in piebaldism (see Chapter 138), thus it is possible that activating mutations may influence pigmentation. This remains to be substantiated in the case of mastocytosis. Mastocytosis is reviewed in Chapter 75.
Erythema Dyschromicum Perstans
Erythema dyschromicum perstans (EDP), or ashy dermatosis, is an acquired disorder of unknown cause. Both sexes and all age ranges and races may be affected [26]. Clinical features are of asymptomatic ash-coloured (blue/brown-grey) macules, which slowly spread and leave long-lasting discoloration. In the early stages there may be a thin erythematous margin. It is most common on the trunk and limbs but may occur on the face also. Mucous membranes are spared. Lesions may be permanent but in children they may clear spontaneously [27]. There is no clearly effective treatment.
Periorbital Hyperpigmentation
Periorbital hyperpigmentation may affect the skin of the upper and/or lower eyelids and adjacent areas. This may be an inherited condition [28]. Periorbital hyperpigmentation has been reported in naevus of Ota [29], erythema dyschromicum perstans [30,31] and hyperthyroidism (Jellinek sign) [32]. Increased pigmentation of the lower eyelids is often seen in atopic individuals (‘allergic shiners’) and may be due to congestion of the nasal and paranasal venous network [33] as a result of chronic allergic rhinitis. In those with atopic dermatitis this may be contributed to by scratching resulting in postinflammatory hyperpigmentation [34]. Contact dermatitis may also result in postinflammatory hyperpigmentation.
Melasma
Melasma is an acquired condition presenting as patches of brown-to-black discoloration on the face. It is significantly more common in women than men [35]. Melasma is uncommon in childhood and early adolescence in most areas, although in parts of India, Pakistan and the Middle East the problem may develop before puberty [36]. It is more common in skin types V–VI [37] and in Hispanics [38]. Melasma may begin during pregnancy in many cases and is also known as ‘the mask of pregnancy’. Hormonal influences may be important in its causation although in affected males this is not the case and ultraviolet exposure seems more significant [35]. Ultraviolet light may also play a significant role in its aetiology in females [35,37,39,40]. Hormone drugs such as the contraceptive pill can trigger the condition [41] as may antiseizure drugs [42], for example phenytoin [43]. Genetic predisposition is a further important causative factor [36,40]. The term chloasma (derived from a Greek word meaning to be green) is used interchangeably with melasma by many, although melasma is a more accurate description of the clinical findings, being derived from the Greek word for black [37].
Pathology.
Melasma is usually divided histologically into one of three forms: a dermal form, an epidermal form and a mixed form [39]. In the epidermal form an increase in melanin is noted in the basal and suprabasal layers but may extend throughout the epidermis. In the dermal form macrophages laden with melanin are noted in the deep and superficial dermis [37].
Clinical Features.
Lesions consist of irregular, macular, brown-to-black patches. Three patterns of distribution predominate. These are centrofacial, malar and mandibular [37]. The centrofacial pattern occurs in around two-thirds of cases and includes forehead, nose, cheeks, upper lip and chin. The mandibular pattern occurs in about 15% of cases. The malar pattern is present in approximately 20% of cases [43]. Skin at other sun-exposed sites such as forearms may be involved regardless of facial pattern. Wood’s lamp examination may indicate an epidermal (darker), dermal (no change), mixed involvement or indeterminate (lesions cannot be seen with Wood’s lamp) pattern [42].
Prognosis.
Once established, melasma may persist for a prolonged duration. After cessation of the oral contraceptive it may remain for more than 4 years [44]. Melasma of pregnancy usually resolves several months after delivery [45] but may recur in subsequent pregnancies. Melasma with dermal involvement is the most difficult pattern to manage, whilst the epidermal pattern tends to respond more favourably to treatment [37,38].
Differential Diagnosis.
Postinflammatory hyperpigmentation due to cutaneous lupus, atopic dermatitis, contact dermatitis, photocontact or photosensitivity reactions may present with similar features but may have a history of an inflammatory phase. Actinic lichen planus may occur in childhood and can result in a similar facial appearance; the histology may differentiate it [46,47]. Where there is a history of hydroquinone use, particularly if melasma is deteriorating, exogenous ochronosis should be considered [48,49].
Treatment.
Sun avoidance and regular use of a broad-spectrum sunscreen is a key part of management [36,50]. This may be difficult or unpopular. Any implicated drug such as an oral contraceptive or phenytoin should be stopped. Hydroquinone is the most commonly used agent [42] and may be used in monotherapy or in combination with a retinoid and/or a corticosteroid, the prototype for this type of combination being Kligman’s solution. It is recommended that hydroquinone on its own should initially be given for a 2-month trial and continued if successful [45]. Azelaic acid has also been demonstrated to be efficacious [51]. Glycolic acid peels have shown some promise, alone or in combination with hydroquinone [52]. Various laser regimes have been used, such as Q-switched Nd:YAG, but rapid recurrence is common and with many lasers postinflammatory hyperpigmentation is a risk [45].
Macular Amyloidosis and Lichen Amyloidosus
Amyloidosis is discussed in Chapter 159. Two types have been associated with hyperpigmentation: macular amyloidosis and lichen amyloidosus. Macular amyloidosis usually presents on the upper back as poorly defined, brownish pigmented patches or linear rippling of the skin with closely aggregated greyish-brown macules [53]. Lichen amyloidosus appears as flesh- or brown-coloured papules, which may coalesce into plaques, and is more common on the legs [54]. Amyloid is deposited in the papillary dermis in both conditions [55]. Both forms may be associated with pruritus and excoriation and there is ongoing debate as to the role of scratching in the causation of lichen amyloidosus [56,57]. The role of rubbing in is becoming apparent in the aetiology of macular amyloidosis [53], and some prefer the term ‘friction amyloidosis’ in place of macular amyloidosis [58,59].
Notalgia paraesthetica [60], friction amyloidosis [58] and friction melanosis [61] have clinical similarities to macular amyloidosis. Macular amyloidosis and lichen amyloidosus are not associated with systemic deposition of amyloid. Lichen amyloidosus is reported in association with type 2A multiple endocrine neoplasia (Sipple syndrome) [62].
Diffuse Hyperpigmentation
Genodermatoses
For familial progressive hyperpigmentation and familial primary cutaneous amyloidosis please see Chapter 159.
Endocrine and Metabolic Disorders
Increased pigmentation may be a feature of a number of endocrinopathies and may assist in diagnosis. Addison disease is the best known example and consists of a diffuse brown pigmentation with accentuation at flexures, sites of trauma, buccal mucosa, lips, genitalia, areolae and palmar skin creases. It may be difficult to discern in darkly pigmented skin. The hyperpigmentation is a result of overproduction of adrenocorticotropic hormone (ACTH) by the pituitary because of failure of negative feedback. The structure of ACTH is closely related to melanocyte-stimulating hormone (MSH) and both these substances stimulate melanocyte activity [63]. The aetiology of Addison’s disease in developed countries is predominantly autoimmune, and it may occur alone or as part of an autoimmune polyglandular syndrome (type I), which often presents in childhood [64,65]. A similar pigmentary change may occur in Cushing disease and in Nelson syndrome [66]. Production of ectopic ACTH may result in hyperpigmentation and is associated with small cell lung cancer in adults. Ectopic ACTH is rare in children but has been reported in thymic carcinoid [67]. The hyperpigmentation that occurs in hyperthyroidism is often compared to Addison disease, but it is more variable, does not involve mucosal surfaces and tends to occur on shins, ankles, dorsal surfaces of the feet and nail beds [68].
It is the associated adrenal insufficiency in the very rare Siemerling–Creutzfeldt form of adrenoleucodystrophy that results in increased circulating levels of ACTH, which in turn causes hyperpigmentation [69]. A rare form of primary adrenal failure, familial ACTH unresponsiveness syndrome, presents with hyperpigmentation in childhood and may be associated with alacrima and achalasia [70]. Congenital adrenal hypoplasia is rare and hyperpigmentation usually develops slowly from several months of age. Extremely rarely, it may present in early infancy or at birth and pigmentation may be very dark [71].
In chronic renal failure hyperpigmentation is common [72] and believed to be a result of failure of the kidney to excrete MSH [73]. Many patients with chronic hepatic disease have a degree of increased pigmentation, often a diffuse pattern. Haemochromatosis is an inherited iron storage disease and usually presents in adulthood; however, haemochromatosis type 2 (autosomal recessive) presents in childhood [74]. The iron deposits result in increased melanin [75]. Secondary haemochromatosis due, for example, to multiple blood transfusions, may also occur in childhood.
In adults with type I Gaucher disease, half of patients have diffuse brown or yellow–brown hyperpigmentation with easy tanning. There are no specific pigmentation patterns in type II (infantile) or type III (juvenile) Gaucher disease [76]. Porphyria cutanea tarda (PCT) may present with pigmentation especially on photo-exposed sites, especially in sun-exposed sites; however, it usually presents in adults and is rare in children.
Drug-Induced Diffuse Hyperpigmentation
The groups of drugs that can produce circumscribed hyperpigmentation can also cause diffuse pigmentary changes.
Minocycline usually results in local pigmentary change, but can produce a diffuse muddy-brown discoloration that is emphasized on sun-exposed areas [77]. Phototoxic reactions, such as with doxycyline, may precede generalized postinflammatory hyperpigmentation.
Chemotherapy drugs that may be used in the paediatric setting, such as cyclophosphamide, daunorubicin and hydroxyurea, may result in generalized hyperpigmentation [78].
Long-standing HIV infection can be associated with diffuse hyperpigmentation and therefore it can be difficult to assess the role of drugs especially in the context of polypharmacy. Azidothymidine (zidovudine) and emtricitabine have been reported to cause such reactions [79].
Dioxins may result in hyperpigmentation and porphyria cutanea tarda [80]. The heavy metals silver, bismuth and arsenic may cause a blue-grey pigmentation [81,82].
Hyperpigmentation Resulting from Nutritional Abnormalities
Several vitamin deficiencies may result in increased cutaneous pigmentation. For example, folate deficiency can result in a greyish brown discoloration on sun-exposed areas [83]. Vitamin B12 deficiency may result in increased pigmentation in flexural areas, palms, soles and in the oral cavity [83]. Deficiency in vitamin B3 (niacin), results in pellagra. This has a variety of cutaneous manifestations including hyperpigmentation, following an erythematous dermatitis, on sun-exposed areas and around the neck (Casal’s necklace) [84]. A shiny appearance of the skin is characteristic. The classic presentation of pellagra is of the three (or four) Ds—dermatitis, diarrhoea and dementia (and death if not treated). In up to one-third of cases the cutaneous signs alone may be present [84]. Protein-energy malnutrition in the form of kwashiorkor may result in hypo- or hyperpigmentation particularly at sites of trauma or pressure [85]. Carotenaemia results from excess intake of carotene-containing foods and manifests as yellow/orange discoloration of the skin, especially on palms, soles, forehead, chin, nasolabial grooves, anterior axillary skin folds and pressure areas. Sclerae and mucosal membranes are unaffected. It is usually seen in infants and results from excess consumption of foods such as carrot, squash, broccoli apricots or egg yolk. It is a benign disorder and resolves upon reduction in carotene intake [86–88]. Other cutaneous and clinical manifestations of nutritional deficiency states are discussed in Chapter 65.
Autoimmune Diseases
Autoimmune disease may result in adrenal failure and can present as hyperpigmentation (see Endocrine and metabolic disorders, above). Systemic sclerosis can produce a variety of cutaneous manifestations including generalized hyperpigmentation, localized hyper- or hypopigmentation and a dyschromic appearance [89,90]. In POEMS syndrome, (polyneuropathy, organomegaly, endocrinopathy, M protein, skin changes), also known as Crow–Fukase syndrome, cutaneous changes include generalized hyperpigmentation, hypertrichosis and skin thickening [91,92]. It occurs mainly in the fifth and sixth decades, although there is a case report of an incomplete variant presenting in adolescence [93]. Hyper- and hypopigmented macules are reported as late-phase reactions adjacent to sclerodermatous changes in the toxic oil syndrome, due to contaminated rapeseed oil, which affected large numbers of people in Spain in the 1980s [94].
Miscellaneous Disorders of Hyperpigmentation
In the neonatal period, a rare complication of cholestasis and phototherapy, bronze baby syndrome, results in a grey-brown discoloration. This condition usually resolves once phototherapy is stopped and cholestasis resolves [95]. Congenital adrenal hypoplasia may present with pigmentary change in neonates and is discussed above. An extremely rare condition, universal acquired melanosis (carbon baby syndrome), presents with generalized deep hyperpigmentation shortly after birth. Histology demonstrates a pattern of single melanosomes within keratinocytes, a pattern only seen normally in darkly pigmented skins [96].
Acropigmentation (acromelanosis or Spitzenpigment) presents with brown discoloration confined to the skin around nail beds and is not progressive [97]. Acromelanosis progressiva, another rare disorder, presents initially in a similar manner; however, pigmentation does not fade in adulthood and spreads to affect additional sites such as trunk and limbs [98].
Diffuse hyperpigmentation has also been described in association with multifocal vascular sclerosis [99] and in a form of pseudoleprechaunism referred to as Patterson’s syndrome [100]. Additional clinical findings in this group of patients include cutis laxa, hirsutism, severe skeletal dysplasia and mental retardation.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








