Measles
Measles, or rubeola, is a highly contagious disease with worldwide distribution that remains a leading cause of vaccine-preventable deaths in children.1 The peak incidence of infection occurs during the winter and spring months in temperate areas.2 The risk of mortality is highest in developing countries, with most deaths due to complications of the disease.3 In the United States, measles-related deaths occur in 1–3 of every 1,000 reported cases.2
Before the development of a vaccine, measles epidemics in the United States usually occurred in preschool and young school aged children.2,4 A successful immunization program for children and adolescents, particularly in urban areas, has resulted in a greater than 99% decrease in the reported incidence of indigenous measles since the vaccine was first licensed in the early 1960s.2 Yet, cases of measles continue to occur as a result of viral transmission from importation into the United States.2
Improved immunization programs in developing countries have also prevented outbreaks and reduced measles-associated morbidity and mortality. From 2000–2008, global mortality attributed to measles decreased by 78%, from 733,000 to 164,000 deaths.5 However, the reduction in mortality has leveled off and concerns exist regarding the ability of many countries with a high burden of measles mortality to continue effective measles eradication strategies.
Measles virus, a member of the Paramyxoviridae family, is a heat-labile virus with an RNA core and outer lipoprotein envelope.2,4 Humans are the only natural hosts of the measles virus.2 Measles is spread by direct or airborne droplet exposure. The incubation period is typically 8–12 days, with patients being contagious from 1 to 2 days before onset of symptoms to 4 days after appearance of the rash.2 Both humoral and cell-mediated immunity are needed to control measles virus infection. Immunoglobulin M (IgM) antibodies are detected initially with onset of the rash, followed by a rise in measles-specific IgG titers. The humoral response controls viral replication and confers antibody protection, whereas the cell-mediated response eliminates infected cells.4 A transient immunosuppression occurs during measles virus infection, causing depressed delayed-type hypersensitivity and T-cell counts as well as an increased risk of bacterial infections.4 This process, as well as long-term immunity against measles, is not well understood but may be due to a weak T helper 1 response to the virus.6 Measles virus may use dendritic cells to target lymphoid tissue (CD150 lymphocytes) and disseminate virus throughout the body.7
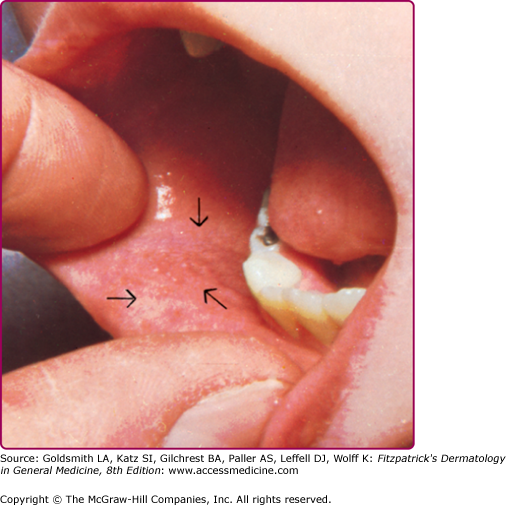
The prodrome is typically characterized by fever (up to 40.5°C), malaise, conjunctivitis (palpebral, extending to lid margin), coryza, and cough (brassy or barking), which may last up to 4 days.2,4 Koplik spots, the pathognomonic enanthem of measles, begin as small, bright red macules that have a 1–2-mm blue–white speck within them. They are typically seen on the buccal mucosa near the second molars (Fig. 192-1 and eFig. 192-2.1) 1–2 days before and lasting 2 days after the onset of the rash (see eFig. 192-1.1).2,4 Individuals with preexisting partial immunity (e.g., patients who received exogenous immunoglobulin) may have less severe symptoms but a prolonged incubation period (14–20 days). Conversely, immunosuppressed patients may have more severe disease and can present without the typical rash.2
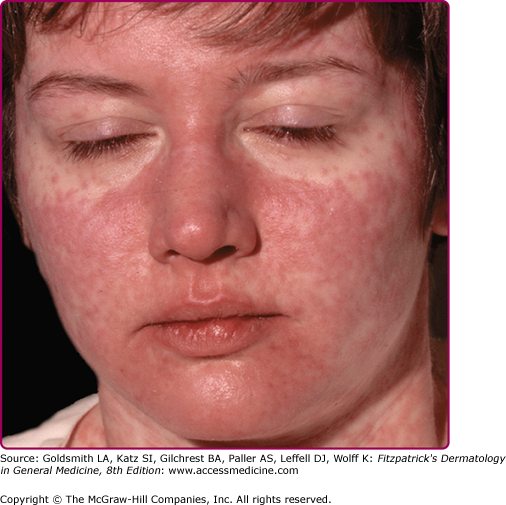
The exanthem is characterized by erythematous, nonpruritic macules and papules that begin on the forehead and behind the ears (Fig. 192-2). The rash quickly progresses to involve the neck, trunk, and extremities (Fig. 192-3 and eFig. 192-3.1). The hands and feet are involved. Lesions may coalesce, especially on the face and neck (see Fig. 192-3.2). The rash usually peaks within 3 days and begins to disappear in 4–5 days. Desquamation may occur as the rash resolves.
The entire illness may last up to 10 days, with some individuals also having vomiting, diarrhea, abdominal pain, splenomegaly, pharyngitis, otitis media, and generalized lymphadenopathy.4 Immunosuppressed patients are at higher risk for pneumonitis, acute encephalitis (1:1000 cases), and other fatal complications.4,8 Viral shedding may also be more persistent in these individuals.2,9
Atypical measles infection is rare, but seen in individuals who received formalin-inactivated measles vaccine (1963–1967) and were subsequently exposed to wild-type virus.4 Symptoms are often more severe with high fever, interstitial pneumonia, pleural effusions, extremity edema, hepatitis, and hyperesthesias occurring more commonly. Coryza, conjunctivitis, and Koplik spots are usually not present. The rash may be maculopapular, hemorrhagic, vesicular, or urticarial and spreads centripetally, making it difficult to distinguish from Rocky Mountain spotted fever.4
The laboratory diagnosis of measles is based on virus detection or positive serologic findings.7 During the prodrome stage, virus can be found in nasopharyngeal secretions, blood, and urine. Viral culture has a low sensitivity for finding the virus but viral immunofluorescence tests may quickly detect measles in throat or nasopharyngeal specimens.10 Indirect enzyme linked immunoassay (ELISA), polymerase chain reaction (PCR), and reverse transcription-PCR assays can detect measles virus in clinical specimens such as nasopharyngeal secretions, oral fluid, serum, dried blood spots collected on filter paper and urine.7,11 Genotyping of viral isolates can determine patterns of transmission and importation. Genome sequencing can differentiate wild type and vaccine virus infection.2
Serologic studies demonstrate measles virus infection with a documented presence of measles IgM antibody and/or a significant increase in measles IgG antibody concentration in paired acute and convalescent titers. IgM antibody increases with the onset of the rash and lasts approximately 1 month. It may be absent or only transient in patients immunized with two vaccine doses.2,12 As the sensitivity of measles IgM assays may vary during the first 3 days after onset of rash, the test should be repeated in any suspected patient with a negative measles IgM assay and a generalized rash lasting more than 72 hours.2 IgG antibody appears 2 weeks after rash onset and peaks 4–6 weeks later.2 In the United States, measles should be reported to the local or state health department.
Most Likely
Consider
Always Rule Out
|
Age-specific rates of complications are highest among children less than 5 years old and adults over 20 years. The most common complications of measles virus infection are otitis media, pneumonia, laryngotracheobronchitis, and diarrhea.2,13 Hepatitis, thrombocytopenia, and encephalitis occur less commonly. Thrombocytopenia-associated purpura may be severe. Pneumonia is the most common fatal complication of measles in children and the most common complication overall in adults.4,14 The disease severity is worse in immunocompromised and malnourished individuals, often resulting in the development of Hecht giant cell pneumonia.15
Subacute sclerosing panencephalitis (SSPE), a rare degenerative central nervous system disease that occurs 7–10 years after wild-type measles infection, is marked by seizures and behavioral and intellectual deterioration.2
Clinical diagnosis of measles is typically made with onset of the characteristic rash as the prodromal symptoms can mimic influenza-like illnesses. Uncomplicated measles is self-limited, lasting 10–12 days. Malnutrition, immunosuppression, poor health, and inadequate supportive care can worsen the prognosis in any patient. In developing nations, measles is a major cause of infant mortality.
Treatment for measles in the majority of cases is supportive, particularly to maintain good hydration. Patients should be on standard and airborne transmission precautions for 4 days after rash onset (entire duration of illness in immunocompromised patients).2 Patients with secondary bacterial infections need to be treated with appropriate antibiotics. Ribavirin may be considered, as it has been shown to inhibit measles virus in tissue culture and reduce the severity and duration of measles in some cases.2,4,16 In trials of patients with subacute sclerosing panencephalitis, however, no benefit was noted with the use of ribavirin.
Malnutrition and vitamin A deficiency can depress cell-mediated immunity in children, increasing the risk and severity of childhood infections. Measles virus infection decreases serum levels of vitamin A and can lead to higher risk of mortality from disease. The World Health Organization (WHO) and the United Nations International Children’s Emergency Fund (UNICEF) issued a joint statement that vitamin A should be administered to all children with measles in communities where vitamin A deficiency is known or where measles mortality is at least 1%.2,17 A 2005 Cochrane review of vitamin A supplementation to treat measles in children found an association between using two doses of vitamin A on two consecutive days and a reduced risk of measles mortality in children less than 2 years old.2,18
All individuals at risk (infants less than 1 year of age, pregnant women, unimmunized, and immunocompromised) should receive immunoglobulin prophylaxis within 6 days of exposure to measles virus.2 If given within 72 hours of exposure, the individual may not be infected with the virus.4 Healthy individuals should receive 0.25 mL/kg of intramuscular immunoglobulin, and immunocompromised patients require 0.5 mL/kg.2 Exposed patients (excluding pregnant women and those with impaired immune systems) should also be given the measles vaccine 5–6 months later to confer lasting protection (Box 192-2).
The incidence of measles has decreased worldwide as a direct result of immunization. Two doses of the live attenuated measles vaccine (with first dose at or after 12 months of age) produces detectable levels of antibody in 99% of individuals, conferring lifelong immunity.2,4 Exposure to measles is not a contraindication to immunization. Measles vaccine may even provide protection in some cases if given within 72 hours of the exposure.2
Common potential side effects of measles vaccine include fever, local injection site reaction and transient morbilliform rash (7–10 days after vaccination; lasts 2 days) that resolve without treatment. Less common adverse reactions include thrombocytopenia and transient neurologic reactions.2,19
Measles vaccine administration is contraindicated in individuals who have a moderate to severe illness as well as those who have had an immediate anaphylactic reaction to a previous measles vaccine. It is also contraindicated in pregnant women and those with impaired immune systems (human immunodeficiency virus infection, immunosuppressive therapy). Hypersensitivity reactions can occur to components of the vaccine such as gelatin, neomycin, or egg white cross-reacting proteins.2,20
There have been many unconfirmed, unsubstantiated but widely publicized reports suggesting a potential link between measles-mumps-rubella (MMR) vaccine and the development of autism and possibly inflammatory bowel disease and atopic disorders. Current scientific evidence does not support a causal link between MMR vaccine and autism, inflammatory bowel disease or atopic disorders.21–25
Rubella
|
Rubella virus has a worldwide distribution with outbreaks occurring most frequently in late winter and early spring months. Humans are the only hosts for infection.26 School-aged children, adolescents, and young adults most often develop the disease. Epidemics occasionally occur in developing countries, especially where vaccines are unavailable.
Since introduction of the rubella vaccine in the United States in 1969, the incidences of rubella and congenital rubella syndrome have drastically declined with no widespread epidemics occurring in the United States. Since 2003, fewer than 20 cases are reported annually in the United States.27 Occasional outbreaks have largely been attributed to failure to vaccinate susceptible individuals. Yet, recent serologic surveys indicate that approximately 10% of the United States-born population >5 years old is susceptible to rubella.26,27 Epidemiologic studies have identified that individuals born outside the country or in a vaccine-poor area also have an increased risk of rubella.26
Rubella is an enveloped positive-stranded RNA virus in the Togaviridae family that is spread through direct or droplet contact from nasopharyngeal secretions.26 Infected individuals shed virus for 5–7 days before and up to 14 days after onset of rash,28 with viremia unlikely after the rash occurs. In most individuals, infection leads to lifelong immunity.
Congenital rubella occurs when a nonimmunized, susceptible, pregnant woman is exposed to the virus. Transplacental infection of the fetus occurs during the viremic stage. The risk is greatest to a fetus exposed to the virus in the first trimester. Congenitally infected infants may shed the virus through urine, blood, and nasopharyngeal secretions for up to 12 months after birth, thus being a potential source of viral exposure to other susceptible individuals.26,28
Primary rubella infection is typically a mild, subclinical disease, particularly in adults.26,28 The prodrome is characterized by low-grade fever, myalgias, headache, conjunctivitis, rhinitis, cough, sore throat, and lymphadenopathy; symptoms that may last up to 4 days and often resolve with appearance of rash. Up to 50% of children with primary rubella infection may have a subclinical infection29 or present only with lymphadenopathy or rash (no prodrome).28 Conversely, older adults may have more severe and persistent prodromal symptoms that may make distinction from rubeola difficult in some situations. The presence of Koplik spots in the mouth favors rubeola. As the prodrome resolves and the rash begins to appear, some patients develop an enanthem consisting of tiny red macules on the soft palate and uvula (Forschheimer spots).30 This enanthem is not diagnostic for rubella.
The exanthem, occurring 14–17 days after exposure, is characterized by pruritic pink to red macules and papules that begin on the face, quickly progressing to involve neck, trunk, and extremities (Fig. 192-4 and “Rubella At a Glance”).28 Lesions on the trunk may coalesce, whereas those on the extremities often remain more discrete. The rash usually begins to disappear in 2–3 days, unlike rubeola, which can be more persistent and clears the head and neck first. Desquamation may follow resolution of the rash.
Lymphadenopathy is usually most severe in the posterior cervical, suboccipital, and postauricular lymph nodes and is noted up to 7 days before the rash appears.26 Enlargement of the nodes may persist for several weeks.
Adults, particularly women (up to 70%), may develop arthritis with rubella infection.26 Both small and large joints may be affected. Joint symptoms often first appear as the rash fades and can last several weeks. In some individuals, the symptoms may become persistent or recurrent. Joint swelling may progress to form a joint effusion.
Diagnosis is typically made using serology to detect rubella-specific IgM antibody (up to 8 weeks after infection) or to document a fourfold rise in antibody titer in acute and convalescent-phase serum.26,30 Hemagglutination inhibition (less common), complement fixation, immunofluorescence assay, and ELISA are some of the methods used to detect antibodies.11 As with measles, rubella cases should be reported to local or state health departments.
Viral culture (nose, throat, blood, urine, CSF, and synovial fluid) is sensitive but often difficult due to the influence of timing, collection procedure and transport on the specimen.11 Reverse transcription PCR may be used to detect rubella virus from throat swab or oral fluid with subsequent genotyping of strains to identify a source during outbreaks.11,31 Complete blood cell count usually shows leukopenia with relative neutropenia. Increased numbers of atypical lymphocytes or abundant plasma cells may be noted as well. Patients with meningeal involvement have lymphocytes in the cerebrospinal fluid (CSF).
Women who are infected with rubella during pregnancy may only exhibit minor clinical symptoms. The effects, however, of rubella infection on the fetus can be profound,28 with the greatest risk of fetal malformation in the early stages of pregnancy. Up to 85% of fetuses exposed to rubella within the first 12 weeks of gestation develop serious sequelae such as: microcephaly with mental retardation, congenital heart disease (ventricular septal defect, patent ductus arteriosus, pulmonary artery stenosis), sensorineural deafness, cataracts, glaucoma, low birth weight, and fetal death.27,28,30 Neonatal manifestations of congenital infection include: growth retardation, interstitial pneumonitis, radiolucent bone disease, hepatosplenomegaly, thrombocytopenia, and dermal erythropoesis “blueberry muffin lesions.”26
Diagnosis of congenital rubella infection is obtained by isolating rubella virus in the throat, cataracts, urine, or CSF of the affected neonate. Serologic testing is not as sensitive but is easily available for confirmatory testing. IgM antibody can be detected from birth to 1 month of age; IgG antibody titers may be stable or increase over several months. Laboratory confirmation of congenital infection in children >1 year old is difficult as viral isolation is rare.26,28
Rarely, rubella infection may lead to encephalitis (1 in 6,000 cases),30 with mortality rates varying from 0% to 50%.30 Other rare complications include: peripheral neuritis, optic neuritis, myocarditis, pericarditis, hepatitis, orchitis, and hemolytic anemia.28 Transient thrombocytopenia has been described in 1 in 3,000 children (usually girls); first appearing within days of rash onset and lasting days to months.28,32 Recently, cases of rubella-associated hemophagocytic syndrome and encephalitis have been described.33,34 Congenital rubella syndrome may predispose patients to developing diabetes mellitus.35
Rubella is typically a self-limited disease. Infants who have congenital rubella are infectious until viral shedding from the nasopharynx and urinary tract ends. The majority of infants (85%) infected in utero excrete virus in the first month of life; 1%–3% continue to excrete virus in the second year of life.26,30 Pregnant women caring for these infants are at risk for developing rubella. Clinical course depends on how severely affected the fetus is from intrauterine infection.
Treatment of primary, uncomplicated rubella is supportive. Standard and droplet precautions are recommended for patients with rubella for 7 days after rash onset.26 In nonpregnant individuals, rubella vaccine administration within 3 days of exposure may theoretically prevent illness, though this is yet to be proven.26 Limited data indicate that intramuscular immune globulin (0.55 mL/kg) as postexposure prophylaxis of rubella-susceptible patients may decrease infection, viral shedding and rate of viremia.26 However, absence of clinical signs after administration of immune globulin in a pregnant woman does not assure that congenital infection did not occur. IgM antibody can be used to detect maternal infection after exposure, even after immune globulin administration.26
Neonates with congenital rubella syndrome require supportive care as well as appropriate attention to significant health issues. These infants are contagious and should be isolated to prevent transmission to susceptible individuals.26,28 Contact isolation is recommended for these infants until they are at least 12 months old or if repeated cultures are negative after 3 months of age.26
Rubella vaccine is typically administered as part of a threefold vaccine (MMR) or fourfold vaccine (MMRV) at 12–15 months of age and again at 4–6 years of age. Seroconversion after a single dose of MMR vaccine occurs in 95% of individuals.26,27 It is imperative that individuals at risk for rubella infection are immunized, such as health care workers, military recruits, college students, and recent immigrants.26 Individuals are considered immune to rubella if they have documented vaccination with a live MMR on or after their first birthday, serologic evidence of rubella immunity, or were born after 1957 (except women of child-bearing age).26,30
Potential adverse reactions to rubella vaccine occur in susceptible individuals and include: fever (6–12 days after vaccine), morbilliform rash, lymphadenopathy, and arthralgia.26 Febrile seizures occur more frequently in children 1–2 years old when receiving the first MMR vaccine.26
Pregnant women should not receive the rubella vaccine due to theoretical risk to the fetus.36 Any woman receiving the rubella vaccine should not become pregnant for 28 days. Infants of vaccinated breast-feeding mothers may become infected with rubella via breast milk. Typically, they develop a mild erythematous exanthem of macules and papules with no serious effects.30 As rubella infection confers lifelong immunity, a woman who is reexposed during pregnancy has a low risk of having her fetus contract congenital rubella. Concern about possible infection can be addressed by looking for IgM antibodies in fetal serum (cordocentesis).
Erythema Infectiosum and Parvovirus B19 Infection
|
Erythema infectiosum (fifth disease) is worldwide in distribution, can occur throughout the year, and can affect all ages. It tends to occur in epidemics, especially associated with school outbreaks in the late winter and early spring. Serologic studies show increasing prevalence of antibodies with age. Various studies indicate that from 15% to 60% of children 5–19 years of age and 30%–60% of adults are seropositive.37 Seroprevalence increases to greater than 90% in the elderly.37 Previous infection with B19 seems to confer lifelong immunity.
The incubation period for erythema infectiosum is from 4 to 14 days.38 After intranasal inoculation of parvovirus-infected serum to healthy volunteers, low-grade fever and nonspecific complaints occurred at the time of viremia, 6–14 days after inoculation, and the rash appeared at day 17 or 18.39 Parvovirus B19 is thought to be transmitted primarily by the respiratory route via aerosolized droplets during the viremic phase, and B19 DNA has been found in respiratory secretions of viremic patients.39 After the rash of erythema infectiosum appears, B19 is not found in respiratory secretions and is usually not present in the serum. This suggests that persons with erythema infectiosum are infectious only before the onset of the rash.
The virus seems to be effectively spread after close contact. The secondary attack rate among susceptible household contacts is approximately 50%. Transmission may occur via blood transfusion, from blood products, and vertically from mother to fetus.40 The transmission of B19 through blood transfusion is particularly problematic because the nonenveloped virus is not killed by the solvent detergents or heat that are used to inactivate HIV and hepatitis viruses.41
The B19 virus belongs to the family Parvoviridae and the genus Erythrovirus.42 B19 lacks an envelope and contains single-stranded DNA. It is the smallest single-stranded DNA-containing virus known to infect humans, measuring 18–26 μm in diameter. Parvoviruses are widespread in veterinary medicine, but animal parvoviruses are not thought to be transmissible to humans.41
The pathogenesis of erythema infectiosum is unknown, but the mechanism may involve immune complexes. The more serious manifestations of parvovirus infection relate to the fact that the virus infects and lyses erythroid progenitor cells. Evidence suggests that the blood group P antigen (globoside) is a receptor of parvovirus. Because some individuals lack P antigen, they are not susceptible to infection with B19.43 In patients with increased red blood cell destruction or loss who depend on compensatory increase in red cell production to maintain stable red cell indices, B19 infection may lead to transient aplastic crisis. Such patients include those with anemia associated with acute or chronic blood loss. When parvovirus infects the erythroblasts in a developing fetus with decreased red cell survival, the result may be hemolysis and anemia.44 Anemia may trigger congestive heart failure, edema (fetal hydrops), and possibly fetal death.
Most infections caused by B19 are asymptomatic and unrecognized. Fifth disease, the most common clinical picture associated with the virus, usually begins with nonspecific symptoms such as headache, coryza, and low-grade fever approximately 2 days before the onset of the rash.45 Patients may have headache, pharyngitis, fever, malaise, myalgias, coryza, diarrhea, nausea, cough, and conjunctivitis coinciding with the rash. Approximately 10% of children with erythema infectiosum develop arthralgias or arthritis. Large joints are affected more often than small joints.45 Occasionally, children may present with chronic joint complaints suggestive of juvenile idiopathic arthritis.46
The characteristic rash begins with confluent, erythematous, edematous plaques on the malar eminences, the “slapped cheeks” (Fig. 192-5). As the facial rash fades over 1–4 days, pink to erythematous macules or papules appear on the trunk, neck, and extensor surfaces of the extremities. These lesions have some central fading, giving them a lacy or reticulated appearance (Fig. 192-6).45 The rash can be morbilliform, confluent, circinate, or annular, and there have been reports of palmar and plantar involvement.38 The eruption typically lasts 5–9 days, but can recur for weeks or months with triggers such as sunlight, exercise, temperature change, bathing, and emotional stress. In some outbreaks, pruritus is a major feature of the rash in children.38
There have been occasional reports of parvovirus B19 associated with vascular purpura, including Henoch–Schönlein purpura.47 An enanthem consisting of erythema of the tongue and pharynx and red macules on the buccal mucosa and palate can occur.
Acute arthropathy is the primary manifestation of B19 viral infection in adults.48 It occurs mainly in women and affects the small joints of the hands and, knees. Other joints, such as the spine and costochondral joints, are occasionally involved. This symmetric polyarthritis is usually of sudden onset and is self-limited but can be persistent or recurrent for months. It may mimic Lyme arthritis or rheumatoid arthritis.
The constitutional symptoms are usually more severe in adults than in children.49 Fever, adenopathy, and a mild arthritis without a rash is the usual course. Women are more likely than men to have joint complaints and rash, whereas men often present with only a flu-like illness.48 Some adults may have fatigue, malaise, and depression for weeks after the infection. Asymptomatic infection can certainly occur in adults as well as in children. In one outbreak, 26% of adults were reported to be asymptomatic.48 Parvovirus B19 can cause numbness and tingling of the fingers with or without other features of fifth disease.50 Pruritus that is sometimes severe can occur with or without a rash. It has been suggested that if pruritus is a complaint in a patient with acute-onset arthritis, parvovirus should be considered as a possible cause.
The rash in adults, if present at all, is usually macular and blotchy or lacy, often on the extremities, and rarely demonstrates the characteristic slapped-cheek appearance.48 Other cutaneous manifestations associated with B19 infection in adults include purpura, vesicles and pustules, palmoplantar desquamation, a morbilliform exanthem with Koplik spots, and livedo reticularis.
In 1990,51 a unique syndrome of pruritic erythema and edema of the hands and feet with petechiae, fever, and oral erosions was described. This rare exanthem, now known as papular purpuric gloves-and-socks syndrome, seems to affect teenagers and adults. However, it can affect children also.52 Patients usually have mild prodromal symptoms of fatigue and low-grade fever, myalgias, and arthralgias. Subsequently, itchy, painful, symmetric edema and erythema of the distal hands and feet occurs.53 Purpuric papules appear on the hands and feet with abrupt demarcation at the wrists and ankles. The enanthem, if present, arises on the lips, soft palate, and buccal mucosa. The syndrome resolves spontaneously within 2 weeks. Although many viruses have been implicated as causative agents for papular purpuric gloves-and-socks syndrome, B19 is the virus proven to cause the syndrome.54 Importantly, papular purpuric gloves-and-socks syndrome is contagious when the eruption is present, in contrast to erythema infectiosum.
In patients with erythema infectiosum, laboratory results are usually normal, including reticulocyte count, hematocrit, and tests of liver and renal function. Patients with aplastic crisis have reticulocytopenia and anemia, the severity of which depends on the degree of underlying anemia. Reticulocytopenia, anemia, lymphopenia, neutropenia, and thrombocytopenia can occur in healthy individuals with B19 infection, although these are usually not significant enough to cause clinical symptoms. The erythrocyte sedimentation rate is rarely elevated, and rheumatoid factor has been positive in some cases of parvovirus-associated arthritis.
Detection of recent infection is usually performed with assays for IgM antibody. Radioimmunoassay or ELISA techniques can detect IgM within a few days after onset of illness. IgM can be measured for up to 6 months in many cases, although there is a decline in titer in the second month after onset. IgG can be identified with the same techniques by the seventh day of illness and lasts for years and is therefore best for documenting past infection. Parvovirus antibody is often not detectable in immunodeficient persons.
Histologic examination of various tissues demonstrates homogeneous, intranuclear inclusions with peripheral condensation of chromatin in erythroid precursor cells.55 Electron microscopy of these inclusions reveals parvovirus-like particles.55 In fetal tissues, a leukoerythroblastic reaction may also be seen. The histopathologic changes in the skin of patients with erythema infectiosum include a sparse superficial perivascular lymphocytic infiltrate that is not considered diagnostic.
PCR is used to detect B19 DNA.56 This technique is considered one of the most sensitive approaches for detection of the virus within a number of different specimens including serum or plasma, amniotic fluid, placental or fetal tissue, or bone marrow. It is considered the test of choice in an immunocompromised patient. Immunohistochemical techniques can be used to detect B19 parvovirus antigen in a number of different tissues.
Most Likely
Consider
Always Rule Out
|
As more attention is focused on parvovirus B19, an increasing number of complications are recognized. Subclinical infection is quite common. However, this virus can be responsible for a variety of hematologic, rheumatologic, and neurologic abnormalities.
Parvovirus B19 is the most common cause of transient aplastic crisis in patients with chronic hemolytic anemias.57 This has been demonstrated in sickle cell anemia, hereditary spherocytosis, heterozygous β-thalassemia, pyruvate kinase deficiency, and autoimmune hemolytic anemia, as well as in other conditions of decreased red cell production or increased red cell destruction. The aplastic crisis may be the initial manifestation of the underlying hematologic disease.58 Patients typically have fever and constitutional complaints, followed 1 week later by fatigue, pallor, and worsening anemia.59 Cutaneous manifestations are rarely seen with the aplastic crisis. The hemoglobin may fall below 4 μg/dL and is not associated with reticulocyte production. Bone marrow examination shows hypoplasia or aplasia of the erythroid series. Red blood cell transfusion may be necessary, and most patients recover in 1 week, although the problem can be fatal if untreated. Transient red cell aplasia can occur in healthy persons without underlying hematologic abnormalities.60 It is likely that the aplasia is missed in individuals without disorders of shortened erythrocyte survival because the hemoglobin does not drop low enough to cause symptoms.
In immunocompromised patients, B19 infection can cause a serious, prolonged anemia from persistent lysis of red blood cell precursors.60 Parvovirus-related chronic anemia has been reported in HIV-infected patients, as well as in transplant recipients and those with congenital immunodeficiencies, acute leukemias, lupus erythematosus, and during the first year of life without immunodeficiency. These patients respond dramatically to intravenous γ globulin, suggesting that antibody is the main defense to human parvovirus infection.61
Fetal infection with B19 may result in either an unaffected fetus or spontaneous abortion (especially in the first half of pregnancy), hydrops fetalis in the second half of pregnancy, congenital anemia, and even late fetal death.62 Nonimmune fetal hydrops is the most common complication of intrauterine infection with B19. Because B19 virus can infect erythroid precursors, extensive hemolysis can occur in the fetus, leading to severe anemia, tissue anoxia, high-output heart failure, and generalized edema. The fetus may show ultrasonographic evidence of subcutaneous edema, ascites, pleural effusion, pericardial effusion, placental edema, and polyhydramnios. The overall risk of fetal death is not clearly known, but recent studies suggest that this risk is approximately 6.5% with maternal infection.62 The risk of fetal death for a woman with unknown serologic status is estimated to be less than 2.5% after a household exposure and less than 1.5% after a significant work exposure. It seems that in B19-infected pregnant women, most fetuses are not infected; if they are infected, usually there is not an adverse outcome.63 Furthermore, approximately one-half of women of childbearing age are immune to parvovirus infection because of prior infection.
Because parvoviruses are known teratogens in animals, there has been much concern about whether they cause birth defects in humans. In sera collected from 253 infants with a wide range of congenital abnormalities, there was no parvovirus-specific IgM detected to suggest recent infection.64 There has been one report of a B19-infected abortus with eye abnormalities,65 and another of an infected abortus with cleft lip, cleft palate, and micrognathia.66 Rare reports describe cardiac abnormalities.67 neurologic problems,68 and “blueberry muffin” nodules.69 It has been concluded from these few studies that parvovirus B19 is not a common cause of birth defects.
There are reports of B19 infection causing encephalitis, meningitis, brachial neuritis, a myasthenia-like syndrome, and motor weakness. Parvovirus infection has been blamed for a granulomatosis with polyangiitis (Wegener’s) illness,70 polyarteritis nodosa,71 Kawasaki disease,72 and a systemic lupus erythematosus-like picture.73 In addition, there are reports of other hematologic complications including idiopathic thrombocytopenic purpura, transient neutropenia, myocarditis, a hemophagocytic syndrome, and the Blackfan–Diamond syndrome.
Parvovirus B19 infection in healthy individuals is self-limited. The eruption of erythema infectiosum and the parvovirus arthropathy usually resolve in 1–2 weeks, but can recur or persist for months. If untreated, transient aplastic crisis can be fatal, but most patients recover in 1 week. Chronic anemia from B19 usually resolves if treated with γ globulin. Fetal hydrops can lead to fetal death if not treated.
There is no specific treatment available for parvovirus B19 infection. Erythema infectiosum is a benign condition, and usually no treatment is necessary. Supportive therapy for relief of fatigue, malaise, pruritus, and arthralgia may be needed. The chronic anemia of persistent B19 infection may be treated successfully with commercially available intravenous immunoglobulin, which contains neutralizing anti-B19 antibodies. Transient aplastic crisis, which can be life threatening, may require oxygen therapy and blood transfusion.
Serologic testing for B19 IgG and IgM should be offered to pregnant women who are exposed to parvovirus B19. Infected pregnant women are followed by frequent ultrasonograms. Evidence of hydrops fetalis warrants umbilical cordocentesis to check for anemia, viral DNA, IgG, and IgM. The management of infected fetuses is controversial. Some physicians advocate observation because spontaneous resolution is common. Fetuses with severe anemia and compromise are usually managed with intrauterine exchange transfusion, but this procedure does carry risk (Box 192-5).
| Supportive treatment of fatigue, malaise, pruritus, arthralgias IV immune globulin Oxygen and/or blood transfusion may be necessary Possible intrauterine exchange transfusion |
There is currently no vaccine to prevent parvovirus B19 infection. Candidate vaccines are in clinical trials.74 It is not known whether immunoglobulin given around the time of exposure prevents infection or alters the course of the disease.
Because patients with erythema infectiosum are no longer infectious by the time they develop the illness, control measures directed toward these individuals are not likely to be effective. If these persons are hospitalized, no special precautions need to be taken. Because the virus is transmitted before the rash appears, the disease is easily spread in situations of close prolonged contact such as schools, day care centers, workplaces, and homes.
Patients with aplastic crisis or immunosuppression with chronic B19 anemia may have high-titer viremia and are particularly infectious. These individuals should be placed in respiratory and contact isolation if hospitalized, and pregnant health care providers should not care for them directly. Hospital workers are at risk of contracting nosocomial infections from these patients75 and could spread the virus to patients if adequate precautions are not taken.
Epstein–Barr Virus
|
Epstein–Barr virus (EBV) is a worldwide pathogen with more than 90% of adults latently infected.76 In developed countries, most cases of primary infection occur during adolescence or early adulthood, and clinically may present as infectious mononucleosis.77 Primary infection may occur during childhood; however, is often subclinical, probably because children are better able to clear the infection. In developing countries, most of the population is infected during childhood, thus infectious mononucleosis is much less common.
EBV is transmitted primarily by saliva through close contact.78 Viral replication occurs during primary infection, allowing infectious viral particles to be shed from the oropharynx. There are also reports of EBV acquired via blood transfusion.79 It is also thought that EBV may be transmitted through breast milk80 and genital secretions.81
EBV, also called human herpesvirus 4 (HHV-4), belongs to the Herpesviridae family and is approximately 180–200 nm in diameter.78 The double-stranded DNA genome encodes approximately 100 proteins and is enclosed within a capsid. The capsid is surrounded by an envelope, composed primarily of glycoprotein gp350. CD21 is present on the surface of B cells and binds with gp350, allowing entry of EBV into the cell.
EBV infects B lymphocytes directly within the oral mucosa or infects mucosal epithelial cells, which then infect the B lymphocytes.82–84 The infected B cells are activated, and their population is expanded. These B lymphocytes allow dissemination of the virus throughout the lymphoreticular system.85 A clonal expansion of cytotoxic T lymphocytes allows recovery from primary infection and is the source of the atypical lymphocytes associated with EBV infection.86 Symptoms likely result from this immunologic response.
EBV establishes an indefinite latent infection within the B cells, during which time low levels of the latent proteins are produced.86 The previously linear DNA forms a circular structure, called an episome. Thereafter, B cells may reactivate and reinfect the oropharynx; replication and shedding of the viral DNA allows transmission of the virus to new hosts.78,85
There is an incubation period of 30–50 days. Primary infection with EBV in adolescents and adults typically results in infectious mononucleosis, or the “kissing disease.” The classic triad of fever, lymphadenopathy, and pharyngitis are noted in more than 50% of patients.78 The fever may range from 37.5°C (99.5°F) to 40.5°C (104.9°F) and lasts 1–3 weeks. Lymphadenopathy is tender and characteristically found in the posterior cervical chain. Examination of the posterior pharynx may vary from mild erythema to grossly enlarged tonsils with white exudates.85
Seventy percent to 100% of patients with infectious mononucleosis develop an eruption when antibiotics, specifically ampicillin, are administered.83 Similar rashes have been reported with other antibiotics, such as amoxicillin, cephalexin, erythromycin, and levofloxacin. The rash typically begins 7–10 days after the initial administration of antibiotics. A pruritic, erythematous, morbilliform, or scarlatiniform eruption is noted on the trunk and extremities. The rash has been described as copper colored. Coalescence may be observed on the extensor surfaces and dependent areas. (Fig. 192-7 and see eFig. 192-7.1). The palms, soles, or oral mucosa may be involved. Clearance, which is usually within 7 days,87 is accompanied by prominent desquamation.86 The rash is thought to be a result of EBV-induced antibodies that are produced in response to the administered drug; these antibodies subsequently form immune complexes, which fix complement.83 This exanthem does not usually indicate a permanent allergy to the medication.87
An exanthem due to EBV alone occurs in a minority of cases (approximately 5%–15%),83,87 and can similarly be morbilliform and pruritic; however, usually it commences during the first few days of illness and resolves faster, typically in 1–6 days.86,87 Periorbital and eyelid edema may be seen in up to 50% of those with infectious mononucleosis.89 In approximately 25% of cases, an enanthem is noted. Six to 20 petechiae, measuring 0.5–1.0 mm, may be visualized at the junction of the hard and soft palate. Lesions may coalesce forming larger lesions.
Erythema multiforme, erythema nodosum, acrocyanosis, erythema annulare centrifugum, pityriasis lichenoides, palmar dermatitis, cold urticaria, and granuloma annulare have all been reported during infection with EBV.86
Genital ulcers may occur during primary infection with EBV (Fig. 192-8).90,91 They may occur prior to the onset of other features of infectious mononucleosis or occasionally after the fever and lymphadenopathy.92 They are well circumscribed, painful, and deep with a red-violaceous border. The base of the ulcer can be clear, seropurulent, or with granulation tissue. They frequently occur on the labia minora.93 If bilateral, they are often symmetric with a “kissing” pattern. It is unclear at this point whether the ulcer is caused directly by the virus or whether it is due to a host immune response. Biopsy is typically nonspecific and nondiagnostic. The ulcers heal in approximately 2–3 weeks, and treatment is symptomatic.
EBV is currently the most common cause of Gianotti–Crosti syndrome.94 In children, primary infection with EBV is often mild or asymptomatic; as a result, typical features of infectious mononucleosis are usually not observed.86
Associated symptoms and signs may include fatigue, rigors, headache, and hepatosplenomegaly.95
Most patients with infectious mononucleosis demonstrate lymphocytosis, with lymphocytes often accounting for more than 50% of the absolute white blood cell count.96 Atypical lymphocytes are a hallmark, and typically represent more than 10% of total lymphocytes; most of these cells represent activated T cells responding to infected B cells.78 Mild, transient neutropenia97 and thrombocytopenia98 are common. Transaminases are elevated in more than 80% of patients with infectious mononucleosis.99
The presence of heterophile antibodies confirms most cases of infectious mononucleosis.100 Heterophile antibodies recognize antigens on the erythrocytes from a number of different species, including horses, sheep, bulls, and humans; however, they do not recognize EBV. They are thought to be a result of polyclonal stimulation during the viral infection,101 and can occasionally be detected (false positives) in other illnesses such as lymphoma, hepatitis, or autoimmune disease.100 Heterophile antibodies typically appear within 1 week of the onset of symptoms, peak within 2–5 weeks, and may persist for up to 1 year. They are not the ideal diagnostic test in children less than 4 years old, as sensitivity is quite low.95 In the past, the Paul–Bunnell test had been used with sheep erythrocytes.100,96 Currently, the more sensitive monospot test is used, which is a latex agglutination assay with horse erythrocytes to look for heterophile antibodies. Up to 10% of patients with EBV mononucleosis never develop heterophile antibodies; this is referred to as heterophile-negative mononucleosis.
A number of antibodies specific to EBV develop in the course of disease as well.100,95 Measurement of these specific antibodies may be warranted if infectious mononucleosis is suspected and heterophile antibodies are negative. IgM and IgG antibodies form to viral capsid antigen (VCA) and are both present at onset of disease. IgM VCA antibodies wane after approximately 3 months; IgG VCA antibodies persist for life and are a marker for previous infection. IgG antibodies to EBV nuclear antigen develop 6–12 weeks into the course of disease and also persist for life. IgG to early antigen develops at the onset of disease and is made up of two subsets of antibodies, anti-D and anti-R. The presence of anti-D antibodies suggests recent infection, although 30% of patients do not produce antibodies at all. Anti-R antibodies have no clinical significance. Thus, primary infection may be most easily diagnosed in the presence of IgM and IgG VCA antibodies as well as negative IgG EBV nuclear antigen antibodies.102
The morbilliform exanthem associated with EBV demonstrates nonspecific histopathologic findings.103 There is usually a mild perivascular infiltrate of inflammatory cells. Specific cutaneous manifestations may show their characteristic pathologies.
Most Likely
Consider
Always Rule Out
|
Complications of infectious mononucleosis occur in approximately 20% of patients and include airway obstruction, autoimmune hemolytic anemia or thrombocytopenia, neutropenia,85 myocarditis, and hepatitis.80 Neurologic complications occur in approximately 5% of patients.85 Examples include encephalitis, meningitis, and Guillain–Barré syndrome.78 The risk of splenic rupture is estimated at 0.1% and is spontaneous in greater than one-half of cases.104 Between 3% and 30% of patients with infectious mononucleosis also have concomitant group A streptococcal pharyngitis.96 Finally, in some patients, fatigue and hypersomnia can persist up to 6 months.105
Recovery from infectious mononucleosis is typically over 2–3 weeks without specific treatment.100 Disease may be more protracted in older adults. Chronic active EBV infection occurs rarely.78 It begins as a primary EBV infection and persists for more than 6 months with severe illness and histologic evidence for organ disease. EBV DNA or antigens can be demonstrated from tissue, and usually EBV antibody titers are significantly elevated.
Treatment for uncomplicated infectious mononucleosis is symptomatic (Box 192-7).95 Acetaminophen or nonsteroidal anti-inflammatory agents may be useful in treating the fever or throat discomfort. Because splenomegaly is often an associated finding, contact sports should be avoided until the spleen has returned to its normal size to avoid splenic rupture.
First line | Fever, throat discomfort, myalgias, headache. Splenomegaly | Acetaminophen/nonsteroidal anti-inflammatory drugs. Avoid contact sports until spleen size normalizes to prevent splenic rupture |
Second line | Impending airway obstruction, thrombocytopenia, hemolytic anemia | Systemic corticosteroids |
Systemic corticosteroids may decrease the duration of fever or pharyngeal symptoms, but in general, are not recommended for uncomplicated disease.77
There have been complications such as encephalitis or meningitis associated with steroid treatment of infectious mononucleosis. Oral steroids may be considered in patients showing signs of impending airway obstruction, thrombocytopenia, or hemolytic anemia.
A meta-analysis of five randomized controlled trials evaluating acyclovir in the treatment of infectious mononucleosis showed that there was decreased oropharyngeal shedding of the EBV; however, there was no overall clinical benefit demonstrated.106 A multicenter, double-blind, placebo-controlled study involving 94 patients with acute infectious mononucleosis revealed that acyclovir and prednisolone also reduced oropharyngeal replication of virus but did not affect duration of symptoms.107
Because infectious mononucleosis is often mistaken for a bacterial infection, antibiotics may initially be used to treat. Antibiotics should be avoided in infectious mononucleosis as an exanthem is much more likely to occur in this setting.
Vaccinations are currently being investigated and may prove to be helpful in preventing EBV infections in the future, particularly in high-risk patients.78
![]() Oral hairy leukoplakia is a manifestation of EBV infection seen primarily in the HIV population.108 It has also been noted in patients who are immunosuppressed for other reasons and rarely, in patients who are not HIV-infected or immunosuppressed. Unique to oral hairy leukoplakia is that the EBV replicates within the infected cells and expresses lytic viral proteins.109,110 White or gray, verrucous plaques with a corrugated appearance are observed on the lateral sides of the tongue, either unilaterally or bilaterally.83,109 Lesions cannot be scraped from the tongue. Less commonly, there is involvement of the dorsal or ventral tongue, buccal mucosa, or soft palate.108 Most cases are asymptomatic, although occasionally soreness or a burning sensation is reported.109 Oral hairy leukoplakia is not a premalignant condition. It is, however, associated with poorer prognosis in HIV disease.
Oral hairy leukoplakia is a manifestation of EBV infection seen primarily in the HIV population.108 It has also been noted in patients who are immunosuppressed for other reasons and rarely, in patients who are not HIV-infected or immunosuppressed. Unique to oral hairy leukoplakia is that the EBV replicates within the infected cells and expresses lytic viral proteins.109,110 White or gray, verrucous plaques with a corrugated appearance are observed on the lateral sides of the tongue, either unilaterally or bilaterally.83,109 Lesions cannot be scraped from the tongue. Less commonly, there is involvement of the dorsal or ventral tongue, buccal mucosa, or soft palate.108 Most cases are asymptomatic, although occasionally soreness or a burning sensation is reported.109 Oral hairy leukoplakia is not a premalignant condition. It is, however, associated with poorer prognosis in HIV disease.
![]() Histologically, lesions demonstrate parakeratosis, acanthosis, and little to no submucosal inflammation.111 There is association with candidiasis in approximately 70% of cases. Intranuclear inclusions may be seen in the stratum spinosum, and the cytoplasm may show a ballooned or ground glass appearance.
Histologically, lesions demonstrate parakeratosis, acanthosis, and little to no submucosal inflammation.111 There is association with candidiasis in approximately 70% of cases. Intranuclear inclusions may be seen in the stratum spinosum, and the cytoplasm may show a ballooned or ground glass appearance.
![]() Clinical and histopathologic findings are suggestive of the diagnosis; however, the presence of EBV must be demonstrated for definitive diagnosis.109 Accurate diagnosis is particularly important, as oral hairy leukoplakia may be a clinical indicator of immunosuppression, namely HIV disease. The biopsy specimen may be used to identify EBV DNA by in situ hybridization.109,112 Because a biopsy may be contraindicated in certain patients (i.e., children or those with a bleeding disorder), noninvasive techniques for identification of EBV DNA have been studied. Epithelial cells may be swabbed and evaluated via in situ hybridization with high sensitivity.112 Polymerase chain reaction (PCR) analysis of exfoliative cytology samples has been used but has a low specificity.113
Clinical and histopathologic findings are suggestive of the diagnosis; however, the presence of EBV must be demonstrated for definitive diagnosis.109 Accurate diagnosis is particularly important, as oral hairy leukoplakia may be a clinical indicator of immunosuppression, namely HIV disease. The biopsy specimen may be used to identify EBV DNA by in situ hybridization.109,112 Because a biopsy may be contraindicated in certain patients (i.e., children or those with a bleeding disorder), noninvasive techniques for identification of EBV DNA have been studied. Epithelial cells may be swabbed and evaluated via in situ hybridization with high sensitivity.112 Polymerase chain reaction (PCR) analysis of exfoliative cytology samples has been used but has a low specificity.113
![]() Differential diagnosis of oral hairy leukoplakia includes entities such as candidiasis, lichen planus, white sponge nevus, oral graft-versus-host disease, and keratoses due to smoking or friction.109
Differential diagnosis of oral hairy leukoplakia includes entities such as candidiasis, lichen planus, white sponge nevus, oral graft-versus-host disease, and keratoses due to smoking or friction.109
![]() Antiviral agents, topical vitamin A, topical podophyllin, antifungal therapy, cryotherapy, and surgical excision have all been used as treatment regimens; however, therapy in general is not indicated.109 In addition, lesions frequently recur after therapy is discontinued.
Antiviral agents, topical vitamin A, topical podophyllin, antifungal therapy, cryotherapy, and surgical excision have all been used as treatment regimens; however, therapy in general is not indicated.109 In addition, lesions frequently recur after therapy is discontinued.
The latent infection established by EBV has been linked to the development of several malignancies, including nasopharyngeal carcinoma, Burkitt lymphoma, and Hodgkin lymphoma.78 EBV-associated malignancies occur mainly in patients who are immunocompromised due to HIV infection, congenital immunodeficiencies, or those who receive immunosuppressant therapy such as organ transplant recipients. It is thought that the latent infection of B cells allows transformation and immortalization, allowing the development of a neoplasm to occur.114 Different EBV genes are expressed in each type of malignancy and the biology is quite complex.
Nasopharyngeal carcinoma occurs sporadically in the United States and in Western Europe, is common among Alaskan Eskimos, and is endemic in Southern China, Southeast Asia, and in the Mediterranean basin.115 This neoplasm may clinically present with bloody nasal drainage, otitis media, or cranial nerve palsies. Metastasis to the lymph nodes occurs early; thus lymphadenopathy of the head or neck may be the initial presentation. Skin metastases can rarely be the presenting sign, usually appearing as dermal or subcutaneous nodules.116 Clubbing and dermatomyositis have rarely been reported in association with nasopharyngeal carcinoma. EBV is present in the malignant epithelial cells yet is not found in the lymphocytes.78
African Burkitt lymphoma is a mature B-cell neoplasm endemic to Equatorial Africa that involves overexpression of the c-myc oncogene.117 It typically affects children and presents with tumors of the jaw or facial bones.117,118 A sporadic subtype in Western Europe or the United States demonstrates abdominal involvement. The immunodeficient subtype typically occurs in HIV-positive patients and appears most frequently in the abdomen. Virtually all cases of endemic Burkitt lymphoma are associated with EBV as well as a previous malarial infection, whereas the sporadic variant is associated with EBV is less than 20% of cases.118,119 Dissemination to the skin is extremely rare; however, marked lymphadenopathy is commonly observed.118 Biopsy of lesions show a neoplasm of medium-sized cells with abundant basophilic cytoplasm, as well as apoptotic debris and macrophages ingesting apoptotic tumor cells, creating the “starry sky” appearance.
Hodgkin disease is a malignant lymphoma with bimodal age distribution.120 In developed countries, the peaks are in young adulthood and in older adults. In developing countries, children as well as older adults are more frequently affected. EBV is associated with Hodgkin lymphoma in 30%–50% of cases. EBV is linked to Hodgkin disease more commonly in developing countries, in children, and in the setting of immunodeficiency. The majority of patients have a painless mass, frequently in the neck.121 Hodgkin disease has associated skin lesions in 17%–53% of patients.122 The majority of cutaneous findings are nonspecific and considered paraneoplastic in nature. Examples include severe pruritus, eczema, ichthyosis, urticaria, erythroderma, erythema nodosum, and associated mycosis fungoides. Cutaneous infiltration by Hodgkin disease is rarer, and typically occurs in the setting of advanced disease. Findings may include ulcerated nodules, plaques, tumors, papules, or erythroderma. Pathology consists predominantly of an inflammatory infiltrate with scattered characteristic Reed–Sternberg cells, which are CD30+, CD15+, binucleate large cells with eosinophilic nuclei.120 Treatment is radiotherapy, chemotherapy, or a combination.
Certain types of cutaneous T-cell lymphoma have been found to be associated with the EBV. Chronic ulcers, subcutaneous nodules, and violaceous tumors have been noted in patients with angiocentric T/natural killer-cell lymphomas, T large-cell lymphomas, and systemic T-cell lymphoma with cutaneous involvement.123 Necrotic nasal masses, which may affect surrounding skin as well, can also occur in angiocentric T/natural killer-cell lymphomas. EBV may also be associated with subcutaneous panniculitic T-cell lymphoma, which clinically presents as a panniculitis.124 Histologically, there are atypical lymphocytes in the lobules of the fat as well as phagocytizing “beanbag cells” (eFig. 192-8.1).
eFigure 192-8.1
A. Bean-bag cells containing multiple nuclei in a patient with EBV-associated subcutaneous lymphoma, ×400. B. In situ hybridization for EBV-encoded RNA of the same subcutaneous lymphoma shows numerous cells expressing EBV RNA stained black, ×400. (Used with permission from Keiji Iwatsuki, MD, Okayama University, Okayama, Japan.)
Vesiculopapular lesions on the face of a child, resembling hydroa vacciniforme, may be associated with EBV and have a malignant potential. Hypersensitivity to mosquito bites with erythematous swellings or ulcers may also be linked to latent EBV infections. This phenomenon may indicate high risk for progression to a malignant neoplasm.
![]() Kikuchi histiocytic necrotizing lymphadenitis, or Kikuchi disease, is a worldwide, self-limited disease that primarily affects women 20–30 years old.125 Patients typically have unilateral lymph node enlargement, most often in the posterior cervical chain, which may be accompanied by fevers, chills, myalgias, malaise, diarrhea, nausea, vomiting, hepatosplenomegaly, or generalized lymphadenopathy.125,126 Abnormal laboratory values may include leukopenia and atypical lymphocytes.126 The etiology is not known, but it is thought that Kikuchi disease occurs in response to an infectious agent. EBV has been implicated as one causative agent. Skin findings have been noted in up to 40% of patients with Kikuchi disease. Cutaneous findings are nonspecific and may include erythematous macules, papules, plaques, nodules, facial erythema or ulcers of the gingivae.125,127,128 Diagnosis is by biopsy of the affected lymph node, which typically shows histiocytic aggregates, atypical lymphoid cells, karyorrhectic debris, and patchy necrosis as well as absence of neutrophils or a granulomatous reaction.123 Pathology of the skin lesions is similar to that of lymph nodes. The disease typically resolves spontaneously after 1–6 months without treatment.126
Kikuchi histiocytic necrotizing lymphadenitis, or Kikuchi disease, is a worldwide, self-limited disease that primarily affects women 20–30 years old.125 Patients typically have unilateral lymph node enlargement, most often in the posterior cervical chain, which may be accompanied by fevers, chills, myalgias, malaise, diarrhea, nausea, vomiting, hepatosplenomegaly, or generalized lymphadenopathy.125,126 Abnormal laboratory values may include leukopenia and atypical lymphocytes.126 The etiology is not known, but it is thought that Kikuchi disease occurs in response to an infectious agent. EBV has been implicated as one causative agent. Skin findings have been noted in up to 40% of patients with Kikuchi disease. Cutaneous findings are nonspecific and may include erythematous macules, papules, plaques, nodules, facial erythema or ulcers of the gingivae.125,127,128 Diagnosis is by biopsy of the affected lymph node, which typically shows histiocytic aggregates, atypical lymphoid cells, karyorrhectic debris, and patchy necrosis as well as absence of neutrophils or a granulomatous reaction.123 Pathology of the skin lesions is similar to that of lymph nodes. The disease typically resolves spontaneously after 1–6 months without treatment.126
Gianotti–Crosti Syndrome
|
Gianotti–Crosti Syndrome, also known as infantile papular acrodermatitis and papular acrodermatitis of childhood, is a common, self-limited dermatosis seen worldwide. It affects infants and children between the ages of 6 months and 12 years with the peak age of presentation being 1–6 years of age. Cases have a seasonal predilection, most often occurring in spring and early summer. In children, there is neither an ethnic nor sexual predilection.129,130 There have been only scattered case reports in adults, exclusively in women.
GCS is a cutaneous reaction pattern associated with viruses, bacteria, and vaccines. The exact pathogenesis is unclear. However, there is some suggestion that immunizations or immune imbalance may enhance the risk of developing the exanthem following certain infections. A recent study showed an increased incidence of a personal or family history of atopy in children with GCS.131
Historically, it was thought to be induced exclusively by hepatitis B, but over time other infectious triggers have been identified. When hepatitis B is the causative agent, subtype ayw is most frequently seen.132 However, with widespread immunization against Hepatitis B, most cases found in Europe, India, the United States, and Canada are not associated with Hep B infection. EBV is the most common cause in the United States and can be due to initial infection or reactivation.133 Other associated viruses include cytomegalovirus, enterovirus, respiratory syncytial virus, rotavirus, adenovirus, echovirus, pox virus, poliovirus, coxsackie virus (A16, B4, and B5), parvovirus B19, HIV, hepatitis A, hepatitis C, mumps virus, HHV-6, vaccinia virus, rubella virus and parainfluenza virus. Bacterial pathogens include Mycoplasma pneumoniae, Borrelia burgdorferi, Bartonella henselae, and group A β-hemolytic streptococcus. Immunizations that have been associated include influenza, diptheria-pertussis-tetanus (DPT), bacillus Calmette–Guérin, Haemophilus influenzae type b, MMR, hepatitis B, Japanese encephalitis and oral polio vaccine. Although many groups have tried using both electron microscopy and immunohistochemistry, neither viral particles nor viral antigens have been demonstrated in skin lesions of GCS.134,135 Thus, today it is accepted that the mechanism of lesion development does not involve a direct local interaction between viral antigens and immune-competent cells inthe skin.
Before the onset of the exanthem, a nonspecific prodrome of upper respiratory tract symptoms with fever and pharyngitis plus lymphadenopathy may be present.
Regardless of the cause, the clinical features in all cases tend to be the same. Typically, patients abruptly develop multiple coalescing, monomorphous, flat-topped or dome-shaped, red–brown papules and papulovesicles (Fig. 192-9 and see eFig.192-9.1 and eFig. 192-9.2). Lesions can be pruritic and, rarely, hemorrhagic. Papules vary from 1 to 10 mm in diameter (see Fig. 192-9B) and are distributed symmetrically on the cheeks, extensor surfaces of the extremities, and the buttocks. The trunk, palms, and soles are usually, but not always, spared. Occasionally, the small papules coalesce into large plaques. Cutaneous lesions evolve over a few days and last for 2–8 weeks.
Constitutional symptoms, including malaise, low-grade fevers, and diarrhea, are sometimes seen at time of presentation, but are usually mild. Patients can develop lymphadenopathy, especially of the cervical, axillary, and inguinal chains.136
In most patients, the diagnosis is established on clinical findings and no further diagnostic testing is necessary. In patients with hepatomegaly, further diagnostic testing, including complete blood cell count and liver enzymes, may be warranted. Lymphocytosis or lymphopenia are common and do not require further diagnostic evaluation. If there are elevations in the liver enzymes, evaluation for hepatitis or EBV should be performed. If hepatitis is suggested, checking anti-hepatitis A IgG and IgM, hepatitis B surface antigen and core antibody, and anti-hepatitis C IgG is indicated.
Most Likely
Consider Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|