FIGURE 12–1 The components of wound bed preparation are expressed in this paradigm of DIME, and can guide the clinician in the principles to facilitate wound healing.
Debridement of necrotic tissue from a wound bed is a key component of preparing a chronic wound for closure and subsequent healing (FIGURE 12-2).3–12 Numerous evidence-based wound care guidelines describe the importance of adequate and repeated debridement as a fundamental intervention for chronic nonhealing wounds. One of these well-known international documents is the European Pressure Ulcer Advisory Panel (EPUAP) and the National Pressure Ulcer Advisory Panel (NPUAP) guideline Pressure Ulcer Prevention and Treatment: Clinical Practice, which states several reasons to debride a chronic wound (TABLE 12-1).13
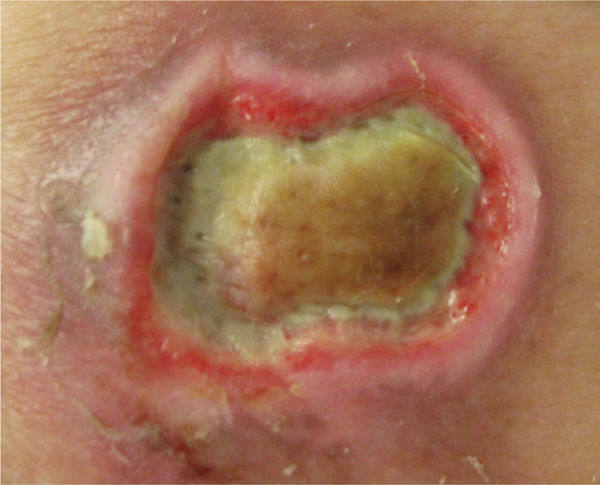
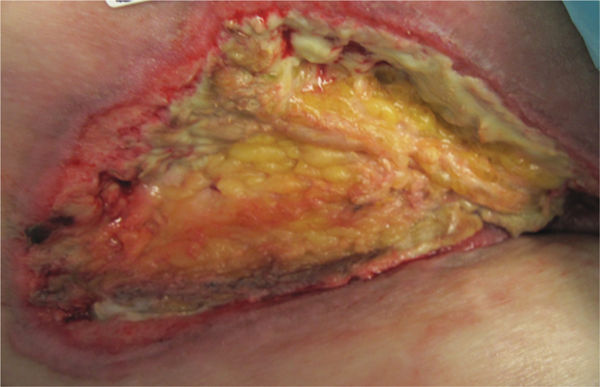
FIGURE 12–2 Wound with necrotic tissue After a partial forefoot amputation, this foot wound has a large amount of necrotic tissue, both eschar and slough, that requires debridement in order for healing to progress.
Table 12–1 Reasons to Debride Chronic Wounds12,13
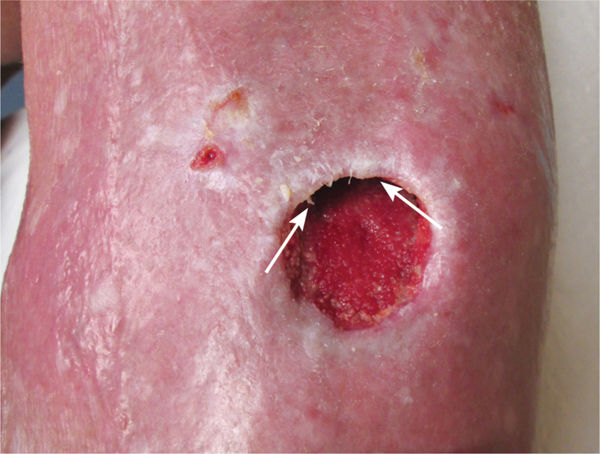
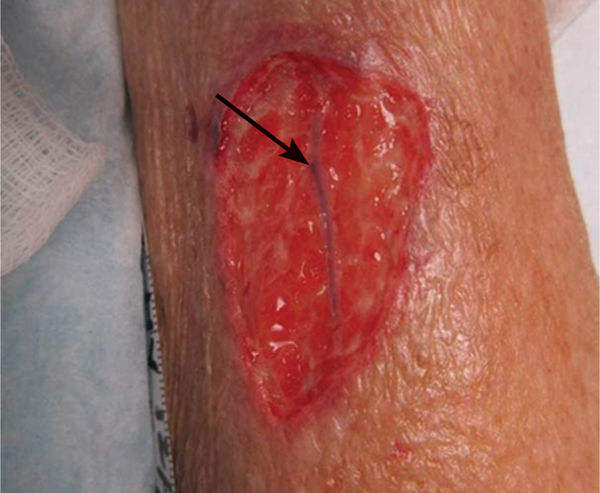
Epibole (FIGURE 12-3) is defined as a condition in which the upper edges of the epidermis migrate down (sometimes with a rolled appearance) to envelop the basement membrane or lower edges of the epidermis. Thus, epithelial cells cannot migrate at wound edges and the wound is unable to resurface.14
FIGURE 12–3 Epibole, as seen between the arrows, will impede wound closure and requires debridement of the edges to facilitate epithelial migration.
During the normal cascade of events leading to wound repair, inflammatory cells such as neutrophils and macrophages are activated to remove microscopic debris, participate in microbial defense, and facilitate the beginning of the repair process. Any interruption in or impairment of the processes associated with the wound repair cascade may create an environment leading to wound chronicity and, ultimately, an environment of increasing necrotic burden.
The term necrotic burden refers to the following components:
 Dead or devitalized tissue
Dead or devitalized tissue
 Excess exudate
Excess exudate
 Higher levels of bacteria.
Higher levels of bacteria.
The necrotic burden is found on the surface of many chronic, nonhealing wounds. In addition, resident cells such as fibroblasts and keratinocytes may be phenotypically altered and no longer responsive to certain signals, such as those from chemical mediators, including growth factors. This state of the cell being alive but impaired by its nonresponsive condition is termed cellular senescence.7,15
TABLE 12-2 lists processes by which necrotic or devitalized tissue impairs and impedes wound closure and healing.
Table 12–2 Ways Necrotic or Devitalized Tissue Impairs and Impedes Wound Closure and Healing
THE DECISION TO DEBRIDE
There are many clinical and patient-centered factors to consider when making decisions about debridement (TABLE 12-3).
Table 12–3 Factors for Consideration Before Debridement2.12.13
The overall condition of the patient and individual goals of care must be considered in the decision to debride.13 Often a terminally ill patient with intact, noninfected eschar may not be a candidate for debridement because of comorbidities, medication, and poor cardiac function. The result of debridement may be a larger, potentially more painful wound requiring more extensive topical care with little or no opportunity for closure due to the patient’s severely compromised medical status. The patient’s general state of health, nutrition and hydration, medications, mobility, and activity status must all be taken into consideration.13 Patients who take anticoagulants may need a less invasive debridement technique than sharp or surgical debridement. (For more information on patient and wound assessment please see Chapter 3, Evaluation of the Patient with a Wound.)
In addition, patients requiring more aggressive techniques are more safely debrided in a controlled setting, such as the operating room or hospital clinic, than at the bedside, in the home, or in the long-term care setting.
Chronic wounds often have underlying pathogenic abnormalities that cause the necrotic burden to reaccumulate. Generally, debridement is not a singular event, but rather combines different methods and/or repeated interventions over time to achieve a clean, functional wound bed. Frequent serial debridement is often necessary to continually stimulate and refresh the surface cells, keeping the wound in a state of readiness to close.15–17
The decision to debride requires careful consideration in certain wound etiologies. Stable, noninfected heel or lower-extremity ulcers with impaired perfusion should not be debrided unless signs of infection are present (eg, erythema, fluctuance, separation of eschar from the edge with drainage, purulence).13,17 Literature states that stable heel ulcers with black eschar should not be debrided13; however, in some instances it may be clinically appropriate to remove stable black eschar in patients who have functional goals and good blood flow. When making the decision to debride stable heel eschar, documentation to support the goals and rationale for performing the procedure are imperative.
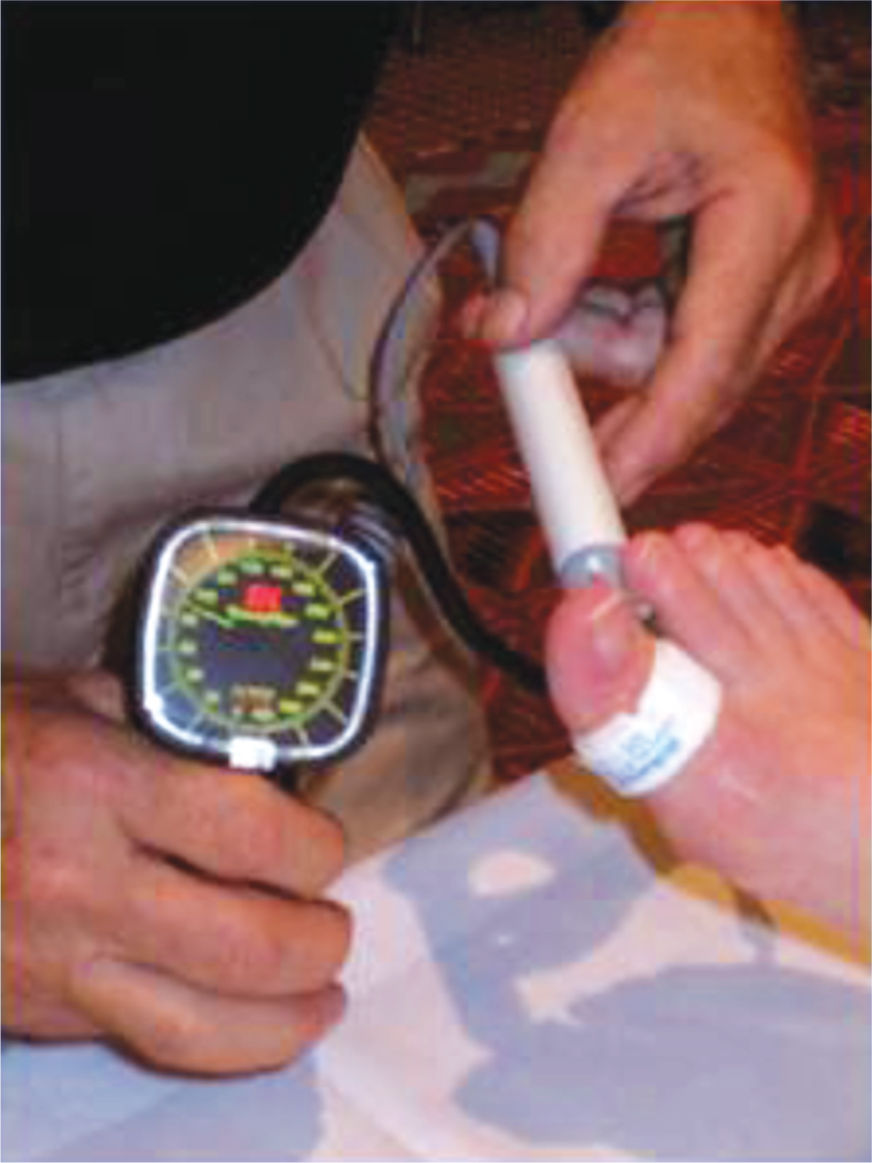
Patients with diabetes who have lower-extremity wounds need more extensive noninvasive vascular assessments—toe pressures (FIGURE 12-5) and wave forms, transcutaneous oxygen tension studies, skin perfusion studies, or Duplex arterial studies—to determine blood flow because of the propensity of the more proximal vessels to calcify in people with diabetes.2,16,19,20 Guidelines for healing potential and for debridement are discussed in Chapter 4, Vascular Wounds.
FIGURE 12–5 Toe pressures in patients with diabetes are assessed before debridement.
Maintaining a dry, stable eschar often leads to slow eschar demarcation and separation as epithelial migration occurs from the edge, eventually closing the wound (FIGURE 12-6). This process has been reported in many cases when a patient is not a candidate for debridement. In addition, diagnoses such as pyoderma gangrenosum may result in pathergy (defined as enlarging or worsening of the wound), with sharp or surgical debridement (FIGURE 12-7).20
FIGURE 12–6 Eschar demarcation and separation Dry eschar on a heel with compromised vascular status is sometimes best left in place and not debrided unless there are signs of infection. The epithelium may migrate from the edges under the eschar, which can then be trimmed as it releases. With meticulous care, these wounds will sometimes progress to full closure, albeit quite slowly.
FIGURE 12–7 Pyoderma gangrenosum Pyoderma grangrenosum has defining violaceous borders around the wound. Wounds suspected of being pyoderma gangrenosum are not sharp or surgically debrided because of the risk of pathergy.
The clinician who performs debridement must have knowledge of anatomy, understand the patient’s overall medical condition, know the wound etiology, and have the technical skills and clinical judgment for debridement, especially when using the more invasive techniques (such as sharp or surgical) in order to have safe, successful outcomes. Clinicians must discriminate viable from nonviable tissue, recognize structures such as arteries and veins, recognize the difference between muscle and granulation tissue, identify fascia covering muscles, identify tendons and cartilage versus slough (which are sometimes similar in color), and identify bone and tendon (see FIGURES 12-8 to 12-17).
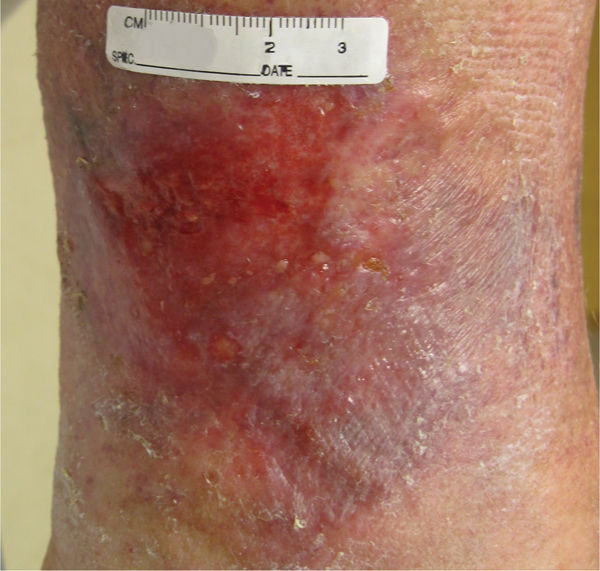
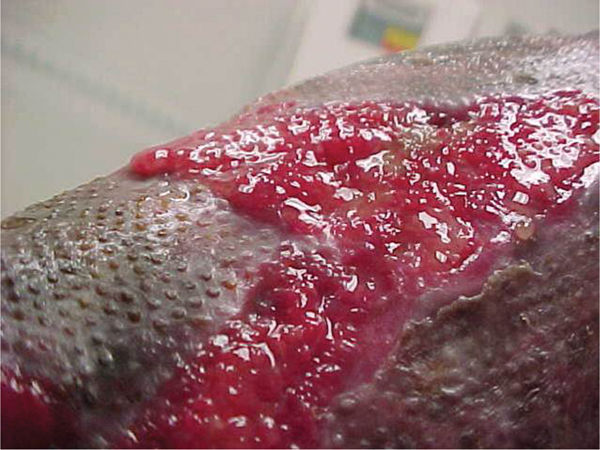
FIGURE 12–8 Healthy granulation Tissue that is pink/red and moist composed of new blood vessels, connective tissue, fibroblasts, and inflammatory cells that fill a healing wound. Typically, appearing with an irregular, bumpy, or granular surface.
FIGURE 12–9 Hypergranulation tissue Granulation tissue that is bulbous and friable suggesting heavy bacterial bioburden. Frequently raised above the level of the periwound skin, it may also be seen in wound base, below the periwound surface.
FIGURE 12–10 Friable granulation A sign of critical contamination, infection, poor extracellular matrix quality, or overall edema.
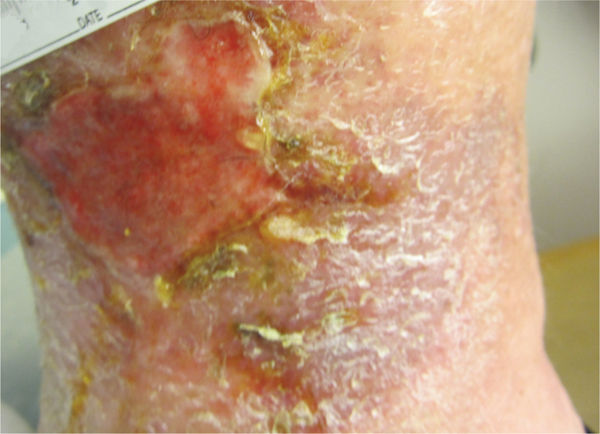
FIGURE 12–11 Slough A metabolic byproduct that is composed of serum and matrix proteins; may be white, yellow, tan, brown, or green. It may be loose or firmly adherent and it has a stringy or fibrous texture and appearance has either a soft mushy or stringy fibrous texture, depending on the tissue that has been autolysed by the phagocytic cells.
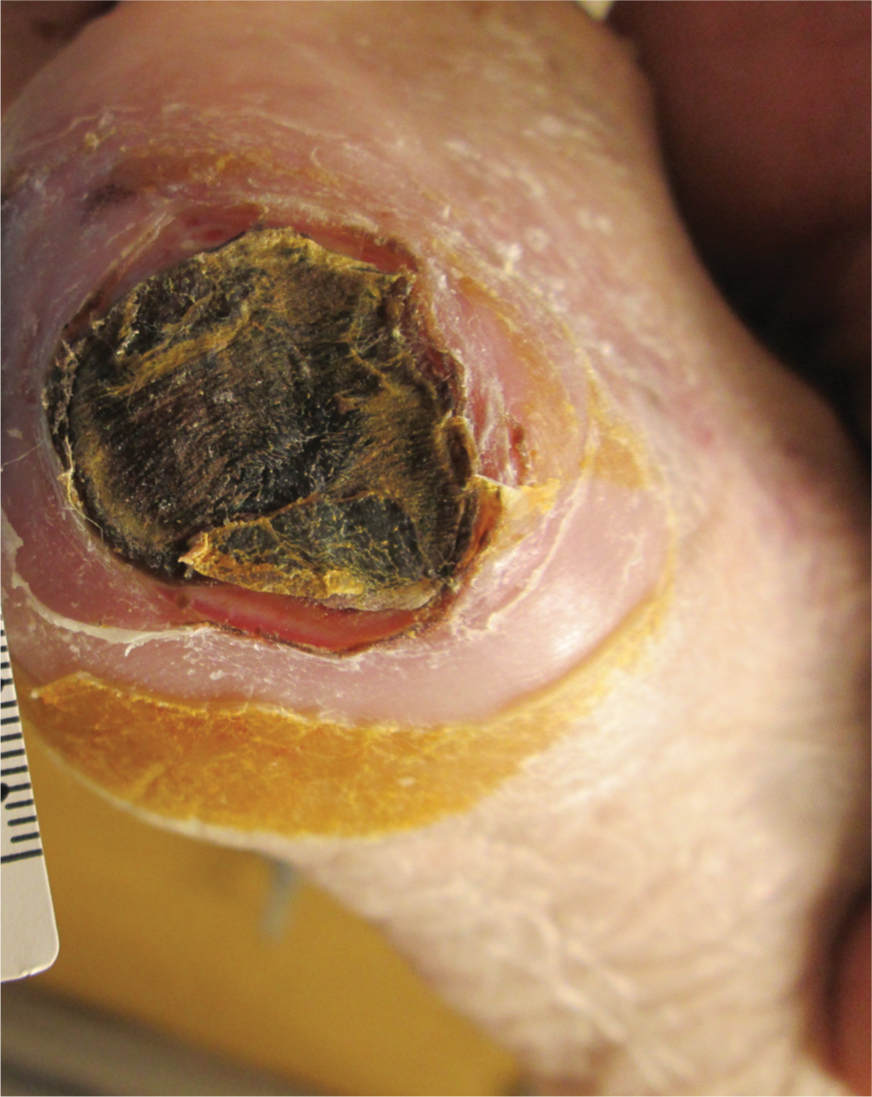
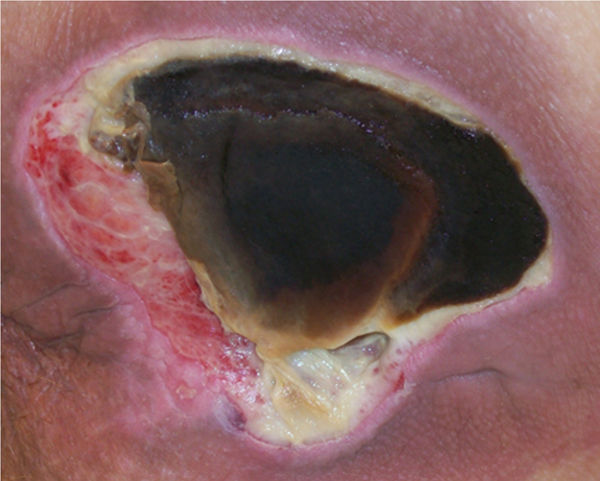
FIGURE 12–12 Eschar Firmly adherent, hard, black, firm, leathery tissue, may be strongly or loosely attached to wound base and edges.
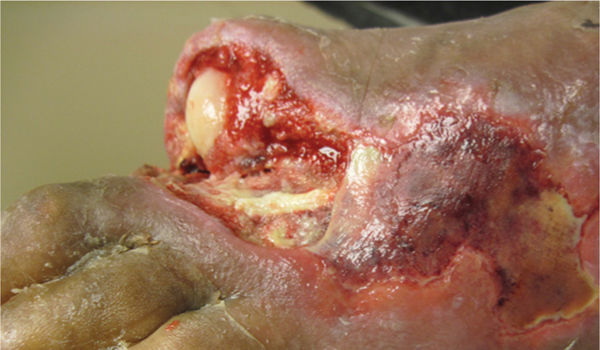
FIGURE 12–13 Vessel in wound bed Any visible vessels, even if they appear coagulated, are to be avoided in order to prevent bleeding.
FIGURE 12–14 Muscle/fascia Healthy muscle is bright red and sensate, bleeds when cut, and may twitch when stimulated. Conversely, necrotic muscle does not bleed when cut, is brown and stringy, and does not contract.
FIGURE 12–15 Subcutaneous fat Fat is poorly vascularized and becomes necrotic and dessicated easier than other tissue types, thus it becomes a likely source of bacteria growth.
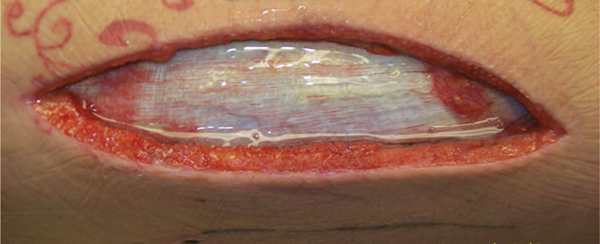
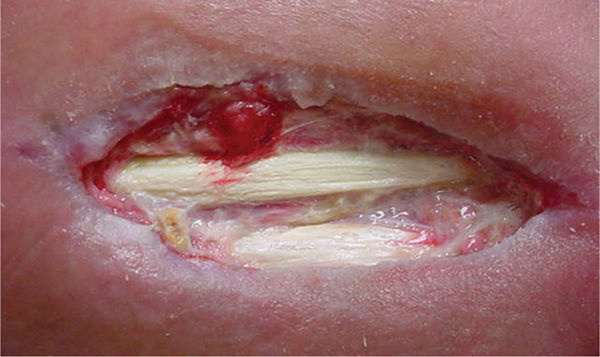
FIGURE 12–16 Tendons Tendon that has the sheath with paratenon intact should be protected with a moist dressing to prevent dessication. Stringy nonviable tendon can be debrided.
FIGURE 12–17 Bone in wound bed
In addition to a thorough awareness of the patient’s clinical condition, knowledge of the different types of necrotic tissue is necessary to determine the most effective type of debridement to use. Necrotic tissue is generally categorized as slough or eschar; however, any tissue described can become necrotic if it loses its blood supply (FIGURES 12-11, 12-12). Eschar may be soft or hard, depending on the amount of desiccation (extreme dryness), and is often described as leathery when completely dry. Slough (FIGURE 12-11) may be yellow, tan, gray, or brown; is generally moist; and may be loosely or firmly adhered to the wound bed. Slough is a by-product of the autolysis of necrotic tissue and as such, usually has a texture that makes it difficult to grasp with forceps.
The presence of granulation tissue is the hallmark of the proliferative phase of wound repair. Healthy granulation tissue most often appears bright or “beefy” red due to its rich vascular composition (FIGURE 12-8). Unhealthy granulation tissue, sometimes called friable or fragile granulation tissue, may be a dull, dark red or a pale, pink color.
FIGURE 12–18 Hypergranulation tissue before treatment with PAV foam
Hypergranulation tissue (also known as “proud flesh,” exuberant granulation tissue, hypertrophic granulation tissue, and hyperplasia of granulation tissue) is a condition that is characterized by an overabundance of granulation tissue, resulting in the extension of this tissue above the level of the wound margins (FIGURE 12-9).18–22 This condition may prevent migration of epithelial cells across the wound surface as these cells do not travel vertically, thereby delaying closure and making the wound more susceptible to infection. In addition, wounds with hypergranulation tissue have an increased risk for excessive scar formation by forcing the wound edges further apart.22 Malignancies can also present with tissue resembling hypergranulation tissue; therefore, an appropriate referral, biopsy, or both may be indicated.22 TABLE 12-42,18,19 provides examples of treatment to diminish or alleviate hypergranulation tissue. FIGURES 12-18 and 12-19 illustrate the use of polyvinyl alcohol foam to treat hypergranulation tissue.
FIGURE 12–19 Hypergranulation tissue after treatment with PAV foam Note that granulation tissue is more flush or even with wound edges, thereby allowing epithelial migration toward the center of the wound bed.
Table 12–4 Examples of Hypergranulation Tissue Interventions 2,21,22
Chapter 3, Evaluation of the Patient with a Wound, provides more detailed information on tissue types in chronic wounds.
TYPES OF DEBRIDEMENT
Debridement falls into two general categories: selective and nonselective. With selective debridement, nonviable tissue only is removed; with nonselective techniques, both viable and nonviable may be removed. Debridement is also classified by the method or the mechanism of action of the various techniques that are used to remove nonviable tissue.
The most common types of debridement used in clinical practice include:
 Autolytic
Autolytic
 Enzymatic
Enzymatic
 Biotherapy or maggot debridement therapy
Biotherapy or maggot debridement therapy
 Mechanical (ie, scrubbing, whirlpool, pulsed lavage, and low-frequency ultrasound)
Mechanical (ie, scrubbing, whirlpool, pulsed lavage, and low-frequency ultrasound)
 Instrument (sharp, surgical, and hydrosurgical)
Instrument (sharp, surgical, and hydrosurgical)
One or more types of debridement may be used over the course of the wound management to achieve and maintain a clean, functional wound bed.
Autolytic Debridement
Autolytic debridement is the process by which the body’s endogenous enzymes (eg, collagenase) loosen and liquefy necrotic tissue in the wound bed (FIGURES 12-20, 12-21). Wound fluid contains these enzymes in addition to inflammatory cells, such as neutrophils and macrophages, that enter the wound site during the normal inflammatory process to remove bacteria and debris.2,14 Some dressing categories customarily used to provide the optimal environment for autolytic debridement include hydrogels, hydrocolloids, transparent films, alginates, medical-grade honey, and polyacrylate moist dressings (see Chapter 13, Wound Dressings).2,18–20
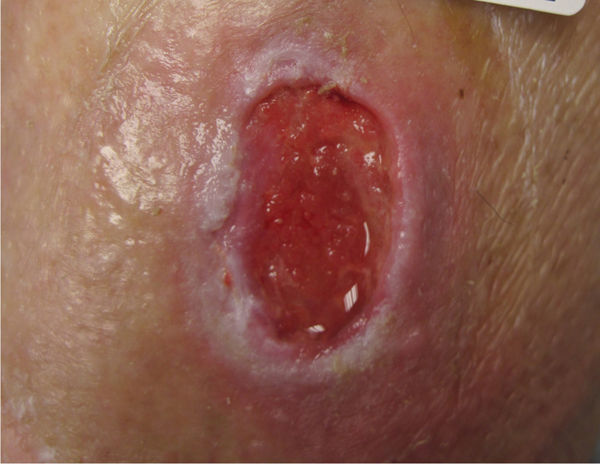
FIGURE 12–20 Wound before debridement
FIGURE 12–21 Wound after autolytic debridement Note the preservation of periwound skin, flat edges, and lack of necrotic tissue, all a result of the patient’s healthy cellular components and enzymes that could break down and phagocytose the nonviable tissue.
The process of autolytic debridement is slower than other methods, requiring multiple dressing applications and frequently taking weeks to complete. Older or frail patients may have difficulty achieving a clean wound bed using autolytic debridement because of their impaired immune system, aging, comorbidities, and nutrition and hydration deficits. This finding suggests that older patients may need more aggressive forms of debridement than the autolytic process alone.23
Autolytic debridement is frequently used in combination with other types of debridement, such as mechanical or instrument debridement. While slower, it may be the least painful type of debridement as long as the patient tolerates the dressing change. Pain control during debridement and dressing changes is a primary consideration. Frequency of dressing changes depends on the amount of nonviable tissue to be removed and the amount of fluid that collects under the dressing.
Fluid collection beneath the selected dressing is monitored to prevent prolonged exposure of intact skin, which could lead to moisture-associated skin damage and ultimately create fragile skin that is at risk for further damage (FIGURE 12-22). Also, the fluid contains dead cells, cellular debris, and bacteria, frequently causing an odor that may lead to the assumption that the wound is infected, despite the absence of other clinical indicators of infection. Culture of the fluid would likely reveal bacteria that are present but not pathogenic to the wound or patient, possibly leading to unnecessary treatment with antibiotics. The wound surface should be thoroughly and safely irrigated or cleansed before assessing for clinical signs of infection.20
FIGURE 12–22 Maceration around the wound edges as a result of exposure to the fluids that collect under the dressing used for autolytic debridement.
Autolytic debridement can be employed to debride wounds of all types, regardless of etiology. However, peripheral arterial disease wounds usually have difficulty autolytically debriding because of the impaired blood supply and consequent lack of moisture in the lower extremity skin.
Autolytic debridement is frequently the initial intervention in the home and long-term care setting where other, more aggressive forms of debridement may not be feasible or available. The only absolute contraindication to autolytic debridement is the infected wound, which needs to be debrided more urgently.13Also, the moisture under the dressing is an ideal environment for bacteria to thrive. Autolytic debridement can be accomplished with minimal technical skills on the part of the caregiver; however, knowledge and skills are required to know if the process is appropriate for a particular wound and patient.
Enzymatic Debridement
Enzymatic, or chemical, debridement uses a topical pharmaceutical preparation that is specifically designed to target and break down the devitalized collagen in the wound bed.14,23–25 As with autolysis, enzymatic debridement is an alternative to the more expensive and aggressive methods of debridement.
At present there is only one commercially available enzymatic debriding agent in the United States, collagenase, marketed under the trade name Santyl (Smith & Nephew, London UK).26 In wound healing, endogenous collagenases belong to a family of extracellular matrix metalloproteases (MMPs) that are naturally occurring enzymes produced by activated inflammatory cells (neutrophils and macrophages) and in certain wound cells (epithelial cells, fibroblasts, and vascular endothelial cells).8,9,25–28
Topical collagenase is derived from the bacteria Clostridium histolyticum. Reported to be most active in a pH range of 6 to 8, topical collagenase is specific to native, denatured collagen.8,15,26 Collagenase promotes debridement by selectively cleaving devitalized collagen strands anchoring necrotic cellular debris without harming viable collagen.8,15,26 Collagenase has no known effect on viable tissue and is not associated with increased patient discomfort.15,26
Collagenase is best applied daily. (FIGURES 12-23, 12-24). Cross-hatching of dry or dense eschar is recommended to facilitate contact and penetration of the ointment and to hasten the debridement process (FIGURE 12-25).20,25 Protection of the surrounding skin with a moisture barrier can prevent maceration and denudation of the intact skin from the increased exudate that results from the enzymatic debridement process. The ointment should not be used with products that could inactivate collagenase, (such as detergents or products containing iodine or heavy metals such as lead, silver, or mercury).26,28
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


 Remove necrotic tissue
Remove necrotic tissue Decrease the bioburden
Decrease the bioburden Remove senescent cells
Remove senescent cells Decrease stimulation of inflammatory cell production
Decrease stimulation of inflammatory cell production Remove callous, rolled edges (epibole
Remove callous, rolled edges (epibole Facilitate angiogenesis
Facilitate angiogenesis Prepare wound bed for skin equivalents/growth factors
Prepare wound bed for skin equivalents/growth factors Prepare wound bed for flap/graft
Prepare wound bed for flap/graft Determine depth of tissue destruction
Determine depth of tissue destruction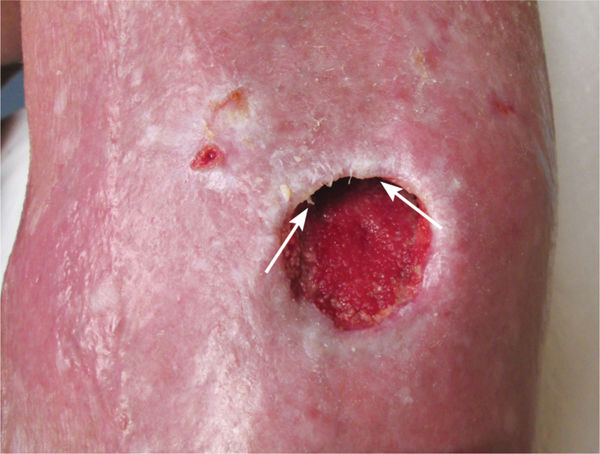
 Masking or mimicking signs of infection
Masking or mimicking signs of infection Serving as a source of nutrients for bacterial cells, thereby contributing to the risk of critical colonization or infection
Serving as a source of nutrients for bacterial cells, thereby contributing to the risk of critical colonization or infection Acting as a physical barrier to closure and impeding normal matrix formation, angiogenesis, or granulation tissue development
Acting as a physical barrier to closure and impeding normal matrix formation, angiogenesis, or granulation tissue development Impairing contraction of the wound area and epidermal resurfacing
Impairing contraction of the wound area and epidermal resurfacing Stimulating the incessant production of inflammatory cytokines, leading to the overproduction of matrix metalloproteases
Stimulating the incessant production of inflammatory cytokines, leading to the overproduction of matrix metalloproteases Overall condition of the patient and their ability to achieve and sustain a closed wound
Overall condition of the patient and their ability to achieve and sustain a closed wound Inclusion of the patient’s and family’s individualized goals for care
Inclusion of the patient’s and family’s individualized goals for care The patient’s ability to adhere to the plan of care
The patient’s ability to adhere to the plan of care Etiology of the wound
Etiology of the wound Type/s of necrotic tissue (ie, slough, eschar)
Type/s of necrotic tissue (ie, slough, eschar) Potential of the wound to close and heal due to local factors (eg, blood flow, presence of infection)
Potential of the wound to close and heal due to local factors (eg, blood flow, presence of infection) Potential of the wound to close and heal due to systemic factors (eg, nutrition, glucose control, immune status, medications)
Potential of the wound to close and heal due to systemic factors (eg, nutrition, glucose control, immune status, medications) Ability to achieve adequate pain control during debridement
Ability to achieve adequate pain control during debridement Clinicians’ knowledge, skills, and expertise
Clinicians’ knowledge, skills, and expertise Available resources to support wound care (eg, personnel, reimbursement, availability of equipment and supplies)
Available resources to support wound care (eg, personnel, reimbursement, availability of equipment and supplies) Vascularity must be assessed prior to performing any debridement of a lower extremity wound.
Vascularity must be assessed prior to performing any debridement of a lower extremity wound. Palpating pedal pulses only of patients with lower-extremity wounds is inadequate for assessing blood flow.
Palpating pedal pulses only of patients with lower-extremity wounds is inadequate for assessing blood flow.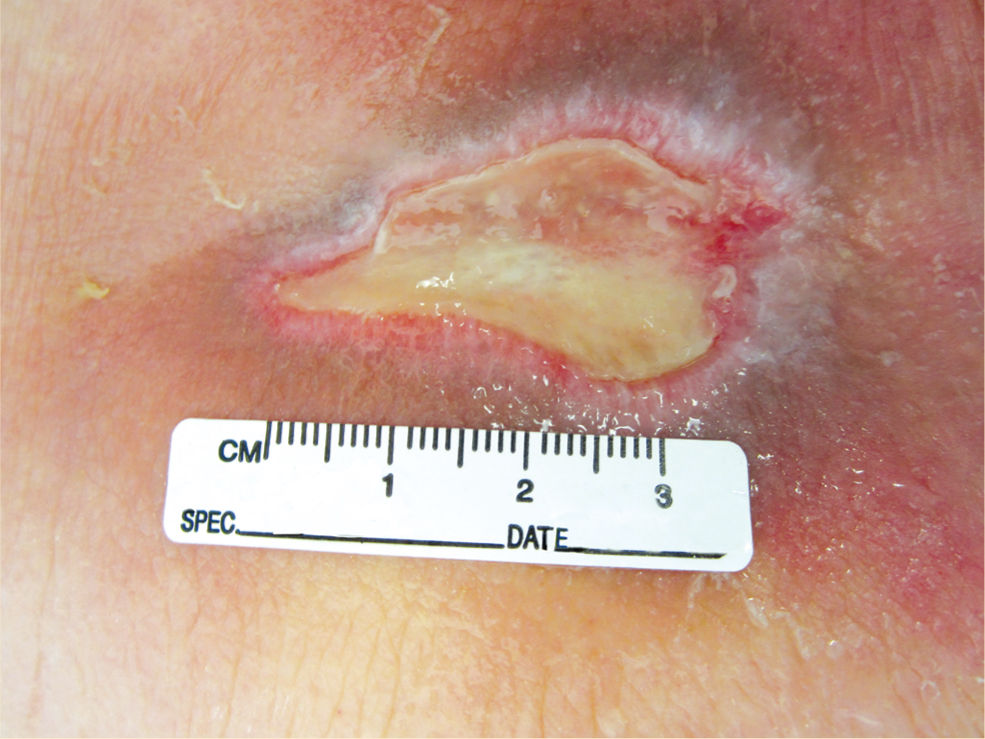
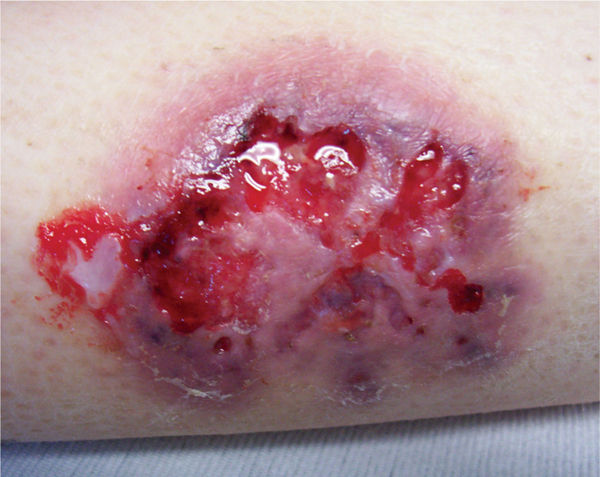
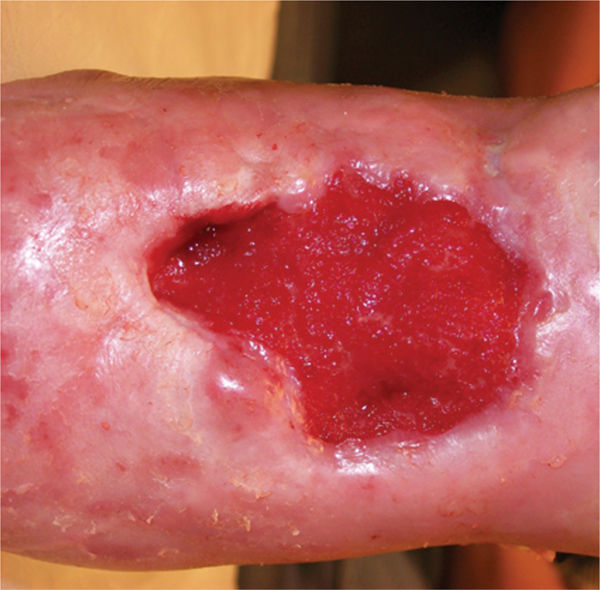
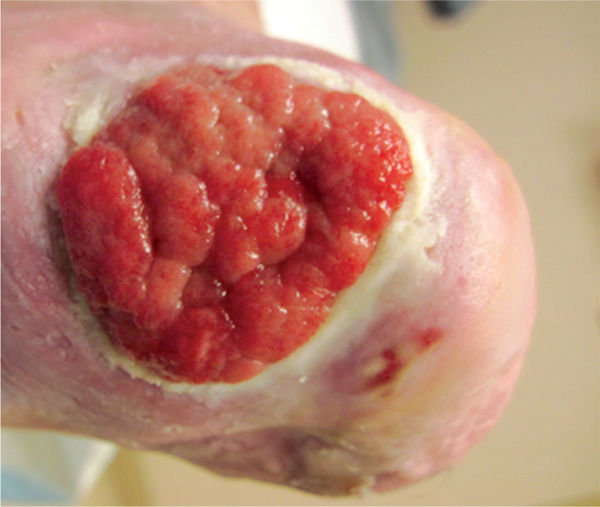
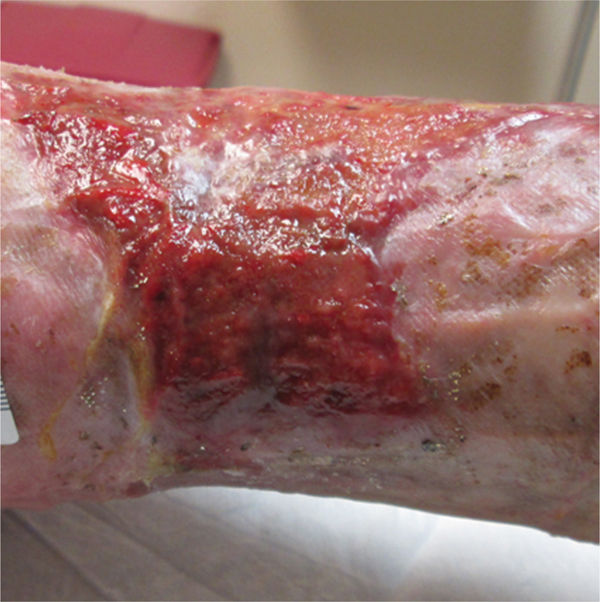
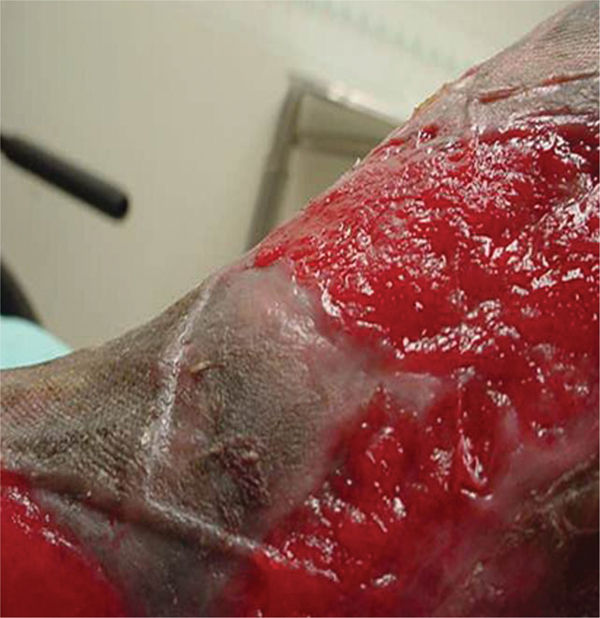
 Compression with dressings such as foams
Compression with dressings such as foams Use of dressings to decrease moisture (eg, hypertonic dressings, calcium alginates, hydrofibers)
Use of dressings to decrease moisture (eg, hypertonic dressings, calcium alginates, hydrofibers) Use of antimicrobial dressings (eg, silver-impregnated dressings, polyvinyl alcohol foam with organic pigments FIGURES 12-18 and 12-19 cadexomer iodine)
Use of antimicrobial dressings (eg, silver-impregnated dressings, polyvinyl alcohol foam with organic pigments FIGURES 12-18 and 12-19 cadexomer iodine) Mechanical debridement (eg, scrubbing, low-frequency ultrasound)
Mechanical debridement (eg, scrubbing, low-frequency ultrasound) Chemical cauterization with silver nitrate
Chemical cauterization with silver nitrate Sharp debridement
Sharp debridement Application of topical corticosteroids
Application of topical corticosteroids Medications: Norvasc, Lipitor, Lasix, two antidepressants, Albuterol
Medications: Norvasc, Lipitor, Lasix, two antidepressants, Albuterol Vital signs (taken at time of evaluation): BP—100/65; HR—78 bpm; O2saturation—94% on room air
Vital signs (taken at time of evaluation): BP—100/65; HR—78 bpm; O2saturation—94% on room air Function: The patient arrives in a wheelchair, records indicate that she is nonambulatory; transfers from bed to wheelchair with moderate assistance and sits in the chair 3-4 hours each morning and afternoon.
Function: The patient arrives in a wheelchair, records indicate that she is nonambulatory; transfers from bed to wheelchair with moderate assistance and sits in the chair 3-4 hours each morning and afternoon. In addition to regular meals, patient receives protein shakes.
In addition to regular meals, patient receives protein shakes.