Introduction608
INTRODUCTION
Post-viral dermatoses
POXVIRIDAE
COWPOX
VACCINIA
Histopathology
Complications of vaccination
VARIOLA (SMALLPOX)
Histopathology
MONKEYPOX
Histopathology
MOLLUSCUM CONTAGIOSUM
Treatment of molluscum contagiosum
Histopathology
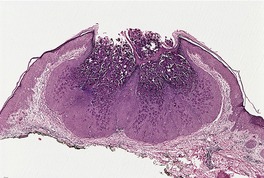
Fig. 26.1
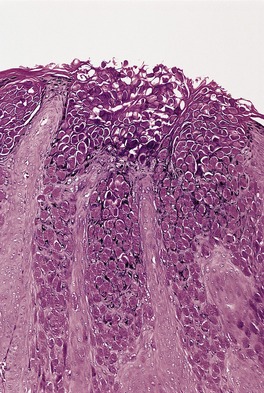
Fig. 26.2
MILKER’S NODULE
Histopathology
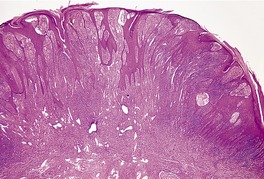
Fig. 26.3
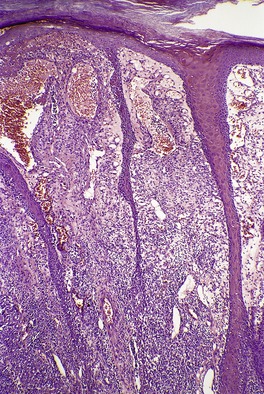
Fig. 26.4
Electron microscopy
ORF
Histopathology
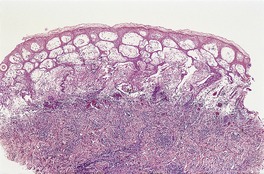
Fig. 26.5
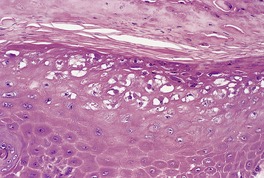
Fig. 26.6
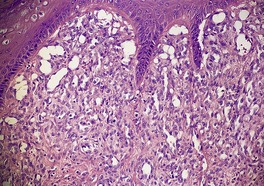
Fig. 26.7
Electron microscopy
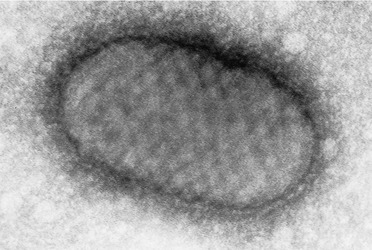
Fig. 26.8
HERPESVIRIDAE
HERPES SIMPLEX
Treatment of herpes simplex
Histopathology
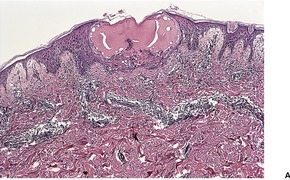
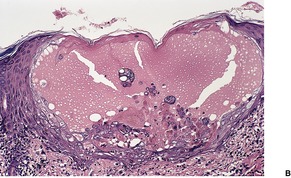
Fig. 26.9
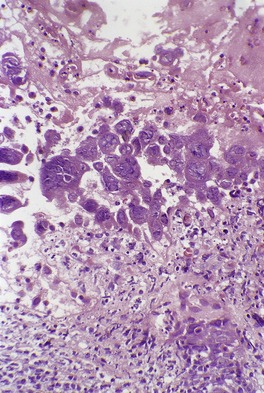
Fig. 26.10
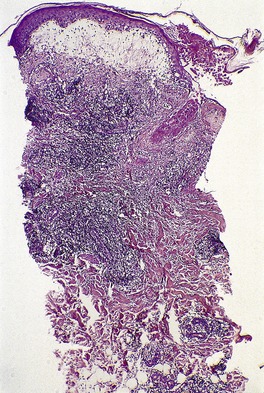
Fig. 26.11
Electron microscopy
Eczema herpeticum
Histopathology
VARICELLA
Histopathology
Electron microscopy
HERPES ZOSTER
Prevention and treatment of herpes zoster
Histopathology
CYTOMEGALOVIRUS
Histopathology
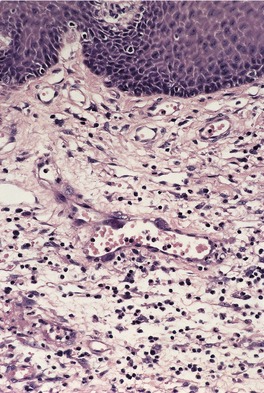
Fig. 26.12
EPSTEIN–BARR VIRUS
Histopathology
HUMAN HERPESVIRUS-6
Histopathology
HUMAN HERPESVIRUS-7
HUMAN HERPESVIRUS-8
PAPOVAVIRIDAE (PAPILLOMAVIRIDAE)
VERRUCA VULGARIS
Treatment of verruca vulgaris
Histopathology510
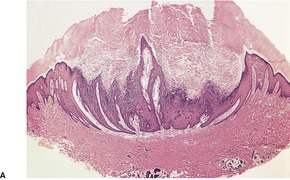
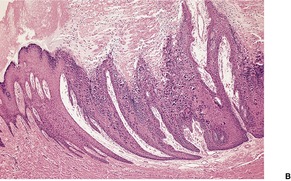
Fig. 26.13
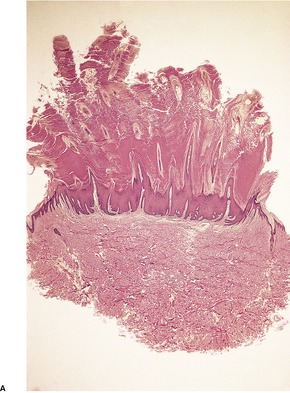
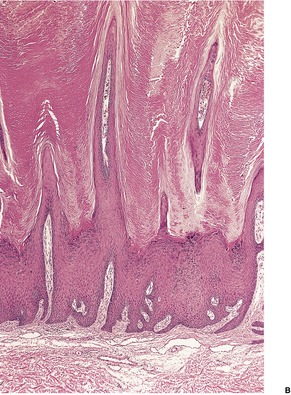
Fig. 26.14
Electron microscopy
PALMOPLANTAR WARTS
Histopathology
VERRUCA PLANA
Histopathology
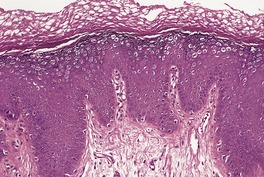
Fig. 26.15
EPIDERMODYSPLASIA VERRUCIFORMIS
Treatment of epidermodysplasia verruciformis
Histopathology609
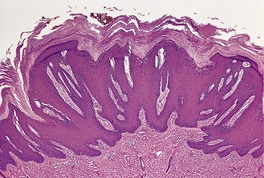
Fig. 26.16
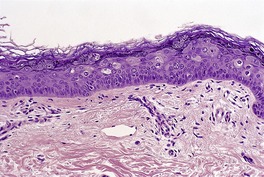
Fig. 26.17
CONDYLOMA ACUMINATUM
Treatment of condyloma acuminatum
Histopathology463
FOCAL EPITHELIAL HYPERPLASIA
Histopathology
BOWENOID PAPULOSIS
Treatment of bowenoid papulosis
Histopathology
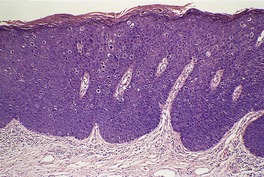
Fig. 26.18
PARVOVIRIDAE
PARVOVIRUS B19
Histopathology744.745. and 747.
PICORNAVIRIDAE
HAND, FOOT AND MOUTH DISEASE
Histopathology
TOGAVIRIDAE
ROSS RIVER/BARMAH FOREST VIRUSES
Histopathology
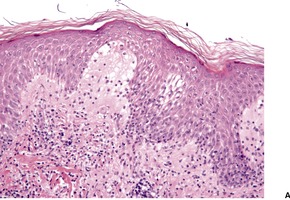
Fig. 26.19
FLAVIVIRIDAE
WEST NILE FEVER/ENCEPHALITIS
Histopathology
DENGUE FEVER
Histopathology
HEPATITIS C VIRUS
PARAMYXOVIRIDAE
MEASLES
RETROVIRIDAE
HUMAN IMMUNODEFICIENCY VIRUS (HIV)
Infections
Viral: molluscum contagiosum, herpes simplex, herpes zoster, verruca vulgaris, condylomas, cytomegalovirus, oral hairy leukoplakia, Kaposi’s sarcoma
Bacterial: mycobacterial infections, more usual bacterial infections, bacillary angiomatosis
Spirochetal: syphilis
Fungal: candidosis, dermatophytosis, histoplasmosis, cryptococcosis, tinea versicolor, phaeohyphomycosis, nocardiosis, mucormycosis, Penicillium marneffei infection
Protozoa: acanthamebiasis, pneumocystosis
Arthropod: scabies, demodicosis
Neoplasms
Kaposi’s sarcoma, cutaneous lymphomas, Bowen’s disease, squamous and basal cell carcinomas, cutaneous melanomas
Dermatoses
Psoriasis, seborrheic dermatitis, pityriasis rubra pilaris, acquired ichthyosis, asteatosis, porokeratosis, vasculitis, folliculitis, contact dermatitis, photosensitivity, vitiligo, yellow nail syndrome, papular eruption, idiopathic pruritus, a chronic diffuse dermatitis, severe drug reactions, alopecia, palmoplantar keratoderma, porphyria cutanea tarda, acrodermatitis enteropathica, neutrophilic eccrine hidradenitis
Histopathology864
HUMAN T-LYMPHOTROPHIC VIRUS TYPE 1 (HTLV-1)
OTHER VIRAL DISEASES
HEPATITIS A VIRUS
HEPATITIS B VIRUS
Gianotti–Crosti syndrome
KIKUCHI’S DISEASE
Histopathology926.935. and 936.
ASYMMETRIC PERIFLEXURAL EXANTHEM
Histopathology
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree



Viral diseases
Viral infections of the skin are of increasing clinical importance, particularly in patients who are immunocompromised. Viruses may reach the skin by direct inoculation, as in warts, milker’s nodule, and orf, or by spread from other locations, as in herpes zoster. Many viral exanthems result from a generalized infection, with localization of the virus in the epidermis or dermis or in the endothelium of blood vessels. 1 The usual clinical appearance of this group is an erythematous maculopapular rash, but sometimes macular, vesicular, petechial, purpuric, or urticarial reactions may be seen. An erythematous-vesicular pattern is more likely to be a viral disease than the other causes of an exanthem such as drugs and bacteria. 2 Some of the varied manifestations of viral diseases may result from an immune reaction to the virus. This is the probable explanation for the erythema multiforme and erythema nodosum that occasionally follow viral infections. Other dermatoses appear to be in this category of a post-viral dermatosis (see below). A new exanthem with a distinctive erythematous macular appearance has recently been described. 3 It is of presumptive viral etiology but no organisms could be detected by the usual means. 3
Viruses are separated into families on the basis of the type and form of the nucleic acid genome, of the morphological features of the virus particle, and of the mode of replication. There are four important families involved in cutaneous diseases: the DNA families of Poxviridae, Herpesviridae, and Papovaviridae, and the RNA family Picornaviridae. In addition to these four families, exanthems can occur in the course of infections with the following families: Adenoviridae, Reoviridae, 4 Togaviridae, Flaviviridae, Retroviridae, Parvoviridae, Paramyxoviridae, Arenaviridae, Filoviridae, and Bunyaviridae.1. and 5. The three major DNA families (there are seven in all) produce lesions that are histologically diagnostic for a disease or group of diseases, whereas the other viruses, particularly the RNA viruses, produce lesions that are often histologically non-specific. These non-specific features include a superficial perivascular infiltrate of lymphocytes, mild epidermal spongiosis, occasional Civatte bodies, and, sometimes, urticarial edema or mild hemorrhage. These features are often given the abbreviated title of morbilliform changes, but they can also be seen in drug eruptions. Although often regarded as favoring a drug reaction, eosinophils can certainly be found in viral exanthems, particularly in older lesions. LeBoit has highlighted these problems in a philosophical article a few years ago. 6 Inclusion bodies, which represent sites of virus replication, are uncommon in skin lesions produced by viruses outside the four major families.
Various laboratory techniques can be used to assist in the specific diagnosis of a suspected viral disease. 7 These include light and electron microscopy of a biopsy or smear, serology, viral culture, and immunomorphological methods. Negative-contrast electron microscopy allows rapid, and virus-family specific, detection of the causative virus. 8 Although viral isolation in tissue culture remains the paramount diagnostic method, the development of monoclonal antibodies to various viruses, for use with fluorescent, immunoperoxidase, and ELISA (enzyme-linked immunosorbent assay) techniques, has made possible the rapid diagnosis of many viral infections with a high degree of specificity. 9 Techniques using the polymerase chain reaction (PCR) are now being used routinely in some laboratories for the diagnosis of certain viral diseases. Serology is still the preferred method of diagnosis for certain viral infections, such as rubella and infectious mononucleosis. Brief mention must be made of the Tzanck smear, which was traditionally used by clinicians, especially dermatologists, in the diagnosis of certain vesicular lesions, especially those caused by the herpes simplex and varicella-zoster viruses. A smear is made by scraping the lesion. This is then stained by the Giemsa or Papanicolaou methods and examined for the presence of viral inclusion bodies. This use is declining with the advent of the more specific immunomorphological techniques.
The various virus families, and the cutaneous diseases they produce, will be considered in turn, after a brief discussion of the concept of the post-viral dermatoses.
Dermatoses are seen, occasionally, which appear to be a reaction to an earlier viral infection. As mentioned above, erythema multiforme and erythema nodosum sometimes follow a viral infection. At other times the viral etiology is presumptive, such as the appearance of skin lesions some days after an upper respiratory tract or gastrointestinal infection of possible viral etiology. Serological evidence of a viral illness, such as IgM antibodies to a particular virus, is sometimes present. Although the dermatoses are thought to result from an immunological reaction to a virus, it does not necessarily persist in the body. There are circumstances in which viral persistence has been demonstrated. Such is the case with the herpes simplex virus and erythema multiforme (see p. 51).
The histological pattern seen in these various post-viral dermatoses is similar – a lichenoid lymphocytic vasculitis. This pattern is characterized by a lymphocytic vasculitis, usually mild and without the presence of fibrin in vessel walls, accompanied by a lichenoid (interface) reaction in which there are variable numbers of apoptotic keratinocytes. Variations on this theme may allow a specific diagnosis to be attached to the process. For example, some cases of pityriasis lichenoides appear to follow a viral illness. On histological examination, there is parakeratosis in addition to a lichenoid lymphocytic vasculitis. In erythema multiforme, associated with herpes simplex, there is a prominent lichenoid (interface) dermatitis with cell death at all layers of the epidermis. In Gianotti–Crosti syndrome (see p. 631), associated with many different viruses, spongiosis is an additional modification to the usual pattern of lichenoid lymphocytic vasculitis.
This concept may need to be modified as additional information becomes available. In the meantime, it provides a plausible explanation of observed cases.
The family Poxviridae is divided into many genera of which the genus Orthopoxvirus includes vaccinia virus, variola virus, cowpox virus, and at least six other species including monkeypox virus, camelpox virus, and raccoonpox virus.10.11. and 12. Only three other genera cause human disease: the genus Parapoxvirus causing milker’s nodule, orf, and sealpox;12.13. and 14. the genus/subgenus Molluscipoxvirus resulting in molluscum contagiosum; 15 and the genus Yatapoxvirus resulting in tanapox. The account that follows will be limited to the following poxvirus infections:
• cowpox
• vaccinia
• variola (smallpox)
• monkeypox
• molluscum contagiosum
• milker’s nodule
• orf.
The causative viruses are large, with a DNA core and a surrounding capsid. There are two subgroups, based on the morphological features of the virus. The viruses of molluscum contagiosum and orf are oval or cylindrical in shape and measure approximately 150 × 300 nm. The remaining viruses are brick-shaped and range in size from 250 to 300 nm × 200 to 250 nm. Clusters of these poxviruses can be identified in hematoxylin and eosin-stained sections as intracytoplasmic eosinophilic inclusions.
Cowpox is a viral disease of cattle. It may be contracted by milkers, who develop a pustular eruption on the hands, forearms or face, accompanied by slight fever and lymphadenitis. Crusted lesions resembling anthrax, 16 and sporotrichoid spread17 have also been reported. A generalized eruption due to cowpox infection may develop, rarely, in patients with atopic dermatitis.18. and 19. This variant, known as Kaposi’s varicelliform eruption, resembles eczema herpeticum (see p. 616). The disease is of historical interest because it was the immunity to smallpox of those who had had cowpox that led Jenner to substitute inoculation with cowpox for the more dangerous procedure of variolation. Doubt has been cast recently on the role of cattle as a reservoir of infection in cowpox.16. and 20. It appears that the domestic cat and rodents have an important role in the transmission of cowpox virus.12.16.19.20.21.22. and 23. Rare cases continue to be reported from Europe24. and 25. and other parts of the world. 26 Rapid identification can be made by an orthopoxvirus-specific PCR. 25
Buffalopox is a similar condition, reported in persons who milk infected buffaloes. 27
Vaccination against smallpox was carried out with the vaccinia virus, a laboratory-developed member of the poxvirus group. In previously unvaccinated individuals, a papule developed on about the fifth day at the site of inoculation. This quickly became vesicular and gradually dried up, producing a crust which fell away, leaving a scar. Exaggerated scarring is a rare complication. 28 Hypersensitivity reactions, which may be exanthematous, urticarial, and erythema multiforme-like, may also develop. 29 Clinically detectable immunity appears to persist for at least 20 years after smallpox vaccination. 30
With the eradication of smallpox, vaccination is no longer given routinely, except to military and key medical personnel. 31 As a result, generalized vaccinia infection (eczema vaccinatum), 32 a serious complication of vaccination, is now of historical interest only. A review of 450 293 military vaccinations performed recently found no resulting cases of eczema vaccinatum and only 21 cases of contact transfer of vaccinia to close contacts. 33 Eczema vaccinatum has many similarities to eczema herpeticum, an infection by the herpes simplex virus seen also in predisposed patients, such as those with an atopic diathesis.
Vaccinia of the labia and inner thighs has been reported as a consequence of conjugal transfer of the virus, from military personnel who received smallpox vaccination. 34 Autoinoculation to another body site is another complication of vaccination. 35
The appearances are similar to those of herpes simplex, zoster, and varicella, except that intracytoplasmic rather than intranuclear inclusion bodies are seen in vaccinia.
Many cutaneous and systemic complications of smallpox vaccination have been reported.36. and 37. They are now of historical interest only, except for late complications, which may continue to be seen. 38
Late sequelae have included keloid formation, basal and squamous cell carcinoma, 39 malignant melanoma, dermatofibrosarcoma protuberans, and malignant fibrous histiocytoma. 40 Dermatoses have also developed, including discoid lupus erythematosus, 41 herpes simplex, 38 lichen sclerosus et atrophicus, 42 contact dermatitis, and ‘localized eczema’. 37 It is possible that some of the late complications represent the chance localization of a particular lesion at the site of previous vaccination.
Variola (smallpox) epidemics have been some of the most deadly to afflict humankind. 31 Epidemics occurred in ancient China, Egypt, and India, and later in the Roman Empire. 31 Variola has now been eradicated. 43 The last known case occurred in Somalia in 1977. Two types were encountered: variola major, a severe form with a significant fatality rate (up to one-third of those affected), 44 and variola minor (alastrim), a mild form with a fatality rate of less than 1%. Umbilicated papules were seen. They became crusted and healed with scarring. The mechanism of the scarring remains speculative but it has been suggested recently that this may have resulted from destruction of sebaceous glands. 45
Variola remains a potential threat in biological warfare as laboratory cultures of the virus still exist. 46
Variola resulted in vesicular lesions that resembled those of herpes simplex, zoster and varicella, except (usually) for the absence of multinucleate epidermal cells and for the intracytoplasmic localization of the inclusion bodies. 47
Human monkeypox, an emerging viral zoonosis, is caused by a member of the genus Orthopoxvirus. It was first identified in the Democratic Republic of the Congo (formerly Zaire) in 1970. It emerged in the United States in 2003, probably as a consequence of the shipment of small animals from Ghana to the United States.48. and 49. Much attention was initially paid to monkeypox because of its clinical similarities to smallpox. 48
In Africa, monkeypox is mainly a disease of children younger than 10 years. Infection is caused mainly by contact with infected small mammals, although human-to-human transmission is not uncommon. 48 Vaccination and vaccinia virus produces about 85% protection against monkeypox. In the cases reported from the United States, all had contact with infected exotic or wild mammalian pets. No human-to-human transmission could be detected. No fatalities were recorded in the United States, although it is 10–17% in African cases. 48
At the edge of the blister there is spongiosis and acanthosis, while the blister itself is an intraepidermal bulla with ballooning degeneration of keratinocytes intertwined with a mixed inflammatory cell infiltrate composed of lymphocytes and neutrophils, with a rare eosinophil. 49 There is progression of the lesion to full-thickness necrosis of a markedly acanthotic epidermis. Rare multinucleated keratinocytes are present but the nuclei do not show viral cytopathic changes. Follicular involvement occurs. 49 A pustular stage then ensues.
Eosinophilic Guarnieri-type intracytoplasmic inclusions are present in affected keratinocytes. A few nuclei may have a central ground-glass appearance mimicking the inclusions of herpesvirus infections, but no true intranuclear inclusions are present. Electron microscopy confirms that the virions are in the cytoplasm of keratinocytes. 49
Molluscum contagiosum is a poxvirus infection of the skin and mucous membranes caused by a virus in the subgenus Molluscipoxvirus, which comprises four genetically distinct, but clinically indistinguishable, viral subtypes. 15 Molluscum contagiosum occurs as solitary or multiple dome-shaped, umbilicated, waxy papules which range in size from 2 to 8 mm in diameter, although solitary lesions may be slightly larger. 50 There is a predilection for the head and neck, trunk, flexural areas, or the genitalia of children and adolescents.15.51.52.53.54.55.56. and 57. Uncommon sites of involvement include the toes, 58 penis, 59 tattoos,60. and 61. burned skin, 62 and herpes zoster scars. 63
Sexual and fomite transmission may occur.64. and 65. Use of swimming pools is associated with some infections. 66 Atopic dermatitis is present in more than 20% of infected patients. 15 One patient with atopic eczema developed erythema multiforme-like targetoid eczema around each lesion of molluscum contagiosum. 67 The coexistence of molluscum contagiosum and verruca plana has occurred in a patient with the hyper-IgE syndrome. 68 Molluscum contagiosum has also developed in patients receiving treatment with tacrolimus or methotrexate, for other diseases.69.70.71. and 72.
Spontaneous regression often occurs within a year, although more persistent lesions are encountered. This regression is primarily a cell-mediated immune response as there is usually an associated lymphocyte response. Pitted scarring is a rare complication of regression in atopic individuals. 73 Antibodies to the virus have been found in nearly 60% of patients with skin lesions, suggesting a role for humoral immunity, but antibodies are less frequent in patients with AIDS. 74 Extensive lesions can occur in immunocompromised patients, particularly those with AIDS.75.76.77.78.79.80.81.82.83. and 84. Disseminated lesions can occur in HTLV-1 infection as well. 85
An additional mechanism appears to be involved in regression. 86 This involves the ubiquitin–proteasome system (UPS). Apoptosis of viral-infected cells appears to be the end-point of these various mechanisms of regression. The virus may try to evade apoptosis by expressing the MC159 protein, which inhibits CD95- and TTVFR-1-induced apoptosis. 86 Apoptosis is not increased in active lesions. 87 Attenuated ubiquitination of molluscum bodies has been reported in a patient with multiple lesions in the setting of malignant lymphoma. 88
The disease is caused by a large brick-shaped DNA poxvirus with an ultrastructural resemblance to vaccinia virus. 89 A well-defined sac encloses the virion colony of each infected keratinocyte. 90 The sequencing of the viral genome is known. 91 The diagnosis can be made in the clinic by the use of 10% potassium hydroxide preparations. 92
The treatment of molluscum contagiosum is varied, with no one modality favored in any country. Solitary lesions are often treated by shave or surgical excision, as the diagnosis may not have been made pre-treatment. Cryotherapy is another option. Sterilized tweezers have been used to remove lesions with some success. 93 Other modes of ‘enucleation’ are favored by some. A prospective randomized trial published in 2006 compared four common treatments: curettage, cantharidin, a combination of salicylic acid and glycolic acid, and imiquimod. 94 Curettage was found to be the most efficacious treatment and had the lowest rate of side effects. However, it is time-consuming and requires anesthesia. Two of the other treatments had side effects considered unacceptable for routine use while the fourth, imiquimod, was said to ‘hold promise’. 94 A study published in 2008, found that systemic drug levels were low in children after single and multiple doses of imiquimod 5% cream. 95 Cidofovir, a drug with broad-spectrum anti-DNA virus activity, has been successfully used in the treatment of recalcitrant lesions in patients with HIV.91. and 96. The injection of candida antigen into the lesion has been used as a form of immunotherapy. Complete resolution occurred in 56% of patients who were followed up. 97
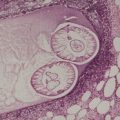
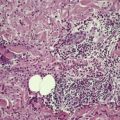
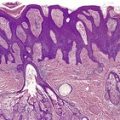
A lesion consists of several inverted lobules of hyperplastic squamous epithelium which expand into the underlying dermis (Fig. 26.1). 89 The lobules are separated by fine septa of compressed dermis. Eosinophilic inclusion bodies form in the cytoplasm of keratinocytes just above the basal layer, and progressively enlarge. At the level of the granular layer, the bodies become increasingly hematoxyphile and occupy the entire cell (Fig. 26.2). These molluscum bodies are eventually extruded with keratinous debris into dilated ostia, which lead to the surface.98. and 99. Areas of hair bulb differentiation, or epithelial proliferation mimicking a basal cell carcinoma, may occur at the margins of a lesion. 98 Molluscum contagiosum has been reported in epidermal cysts,89.100. and 101. but some of these cases may simply represent pilar infundibula dilated by cornified cells and molluscum bodies. 102
Molluscum contagiosum showing inverted lobules of squamous epithelium with molluscum bodies maturing toward the surface. (H & E)
Molluscum contagiosum. The large molluscum bodies occupy almost the whole of each infected cell. (H & E)
Secondary infection and ulceration may occur. 103 Molluscum folliculitis is an uncommon pattern seen mainly in immunocompromised persons. 104 It has also followed leg shaving. 105 The molluscum bodies are present within the follicular epithelium. A variable chronic inflammatory cell infiltrate is seen in regressing lesions, and is thought to represent a cell-mediated immune reaction.98. and 106. However, in the early eruptive phase there is no inflammatory response. 107 Inflammation and a foreign-body reaction may also be related to extrusion of molluscum bodies into the dermis. Rarely, an atypical lymphocytic infiltrate (‘pseudoleukemia cutis’, ‘pseudolymphoma’) may be found.108.109.110. and 111. In one case, the atypical cells were CD8+ T lymphocytes with scattered CD30+ cells. 112 In another they were CD4+, with some CD30+ cells. 110 Another rare inflammatory response is a moderate to heavy infiltrate of eosinophils, with the formation of flame figures.113. and 114.
Molluscum contagiosum has also been reported in association with a nevocellular nevus, a halo nevus,115. and 116. with the Meyerson phenomenon (see p. 717), 117 with cutaneous lupus erythematosus118 and with human papillomavirus (HPV). 119 Co-infection with cryptococcosis has been reported in a patient with HIV infection. 120 In one patient with systemic lupus erythematosus, metaplastic bone was present in the dermis adjacent to each lesion of molluscum contagiosum. 121
Milker’s nodule results from infection with the paravaccinia virus, transmitted from the udders of infected cows. Indirect transmission from contaminated objects has also been reported in several patients with recent burns. 122 Lesions are usually solitary, and on the hands. 123 Multiple nodules and involvement of other sites have been described. Lesions heal, with scarring, in 6–8 weeks. Six clinical stages have been delineated, but the usual lesion, when the patient presents, is a red-violaceous, sometimes erosive or crusted nodule measuring 0.5–2 cm in diameter. 124 Erythema multiforme, erythema nodosum, or urticarial lesions may develop in a small number of cases. 125
The clinical and histopathological similarities between milker’s nodule and human orf have led to the collective term ‘farmyard-pox’ being proposed for these two conditions. 126
Treatment is usually supportive. No trials of antiviral therapy have been published in the dermatological literature. Infection confers lifelong immunity to the host. 123
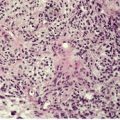
The appearances vary with the stage of the lesion. 124 An early milker’s nodule shows vacuolization and ballooning of the cells in the upper third of the epidermis, leading sometimes to multilocular vesicles. 127 Intracytoplasmic and, rarely, intranuclear inclusions may be seen. 128 Focal epidermal necrosis sometimes occurs, and this may lead to ulceration and secondary scale crust formation. Neutrophils are found in the epidermis and superficial papillary dermis when epidermal necrosis occurs. Mature lesions will show acanthosis of the epidermis, with the formation of finger-like downward projections of the epidermis (Fig. 26.3). There is prominent edema of the papillary dermis, with an inflammatory infiltrate comprised of lymphocytes, histiocytes, plasma cells, and occasional eosinophils. There are numerous small blood vessels, many of which are ectatic, in the papillary dermis (Fig. 26.4). In regressing lesions there is progressive diminution of the acanthosis, and eventually of the inflammatory infiltrate. 124
Milker’s nodule. The elongated thin rete pegs with intervening heavy inflammation of the upper dermis are characteristic features of mature lesions. (H & E)
Milker’s nodule. The dermis between the thin rete pegs is vascular and inflamed. (H & E)
Electron microscopy may show a large oval viral particle with a central electron-dense core surrounded by a less dense homogeneous coat and two narrow electron-dense layers. Rapid diagnosis may be made by electron microscopy of the crust from an early lesion. 129
Orf (ecthyma contagiosum) is primarily a disease of young sheep and goats, involving the lips and perioral area.126.130.131.132.133.134.135. and 136. It is caused by a poxvirus of the paravaccinia subgroup. Orf can be transmitted to humans by contact with infected animals; rarely, lesions have developed at sites of trauma produced by an inanimate object. 135 Lesions, which measure approximately 1–3 cm or more132. and 133. in diameter, develop most commonly on the hands and forearms. Other sites of involvement have included the face,137. and 138. scalp, temple, 139 and perianal region. 134 Several lesions may be present in the one area. Spontaneous regression is usual after about 7 weeks. Recurrent lesions have been reported in immunocompromised persons; 140 such lesions may be quite large. 141 A mature lesion is nodular with central umbilication and an erythematous halo. Regional adenitis, superinfection, toxic erythema, erythema multiforme, widespread lesions, or a generalized varicelliform eruption may complicate the infection. 142 Bullous pemphigoid, mucous membrane pemphigoid, 143 and a subepidermal blistering disease that was thought to be a distinct autoimmune blistering disorder144 may also develop following orf.145. and 146.
As spontaneous regression eventually occurs treatment is not usually given in cases where a clinical diagnosis is made. Many lesions are surgically excised or shaved as a diagnostic tool. As large and atypical lesions may develop in immunocompromised patients, multiple treatment modalities have been used in these patients with variable results. Surgical excision, 40% topical idoxuridine application, cryotherapy, interferon, topical cidofovir, and imiquimod 5% cream have all been used.
The appearances vary with the stage of the disease (Fig. 26.5).130. and 131. Early lesions of orf show moderate acanthosis and pale vacuolated cytoplasm, involving particularly the upper epidermis. 147 Cytoplasmic inclusion bodies are usually present (Fig. 26.6). 126 An unusual change, which has been called ‘spongiform degeneration’, can be seen, particularly in follicular structures. 126 This is characterized by vacuolated cells having wispy strands of eosinophilic cytoplasm. Intraepidermal vesicles or bullae may form. The dermis contains dilated thin-walled vessels and an infiltrate of lymphocytes, macrophages, and occasional eosinophils and plasma cells. 131 Cells expressing CD30 are sometimes present in this infiltrate. 148 Later lesions often show epidermal necrosis, particularly in the center. Neutrophils are often found within and adjacent to the necrotic epidermis. Other biopsies may show elongated rete pegs with dilated vessels in the intervening dermal papillae. Sometimes there is an unusual proliferation of endothelial cells in the dermal papillae which may even simulate a vascular tumor (Fig. 26.7). 139 Eventually the inflammatory infiltrate and epithelial hyperplasia resolve. The lesions of orf are generally regarded as indistinguishable from milker’s nodules, 126 although full-thickness epidermal necrosis seems to be more common in orf. Immunoperoxidase techniques using orf-specific monoclonal antibodies can be used to confirm the diagnosis, if necessary.
Orf. There is epidermal necrosis, acanthosis, and subepidermal edema. (H & E)
Orf. Inclusion bodies are present in the cytoplasm of the keratinocytes in the upper epidermis. (H & E)
Orf. The papillary dermis may be so vascular that a vascular tumor is suspected. (H & E)
Electron microscopy shows an oval virus with an electron-dense core, surrounded by a laminated capsule similar to the virus of milker’s nodule (Fig. 26.8). 149 Rapid diagnosis may be made by electron microscopy of negatively stained suspensions from the lesion. The number of virus-containing cells is greatest in the first 2 weeks of the disease; they may be absent by the fourth week. 150
Orf. Ultrastructural features of the orf virus with its laminated capsule and internal cross-hatched appearance. (Electron micrograph ×20 000)
More than 80 herpesviruses have now been identified, eight of which are known human pathogens. 151 There are three major subgroups within the family Herpesviridae. The α-herpesviruses are neurotropic and include herpes simplex virus (types 1 and 2) and the varicella-zoster virus.152. and 153. The γ-herpesviruses, the second major subgroup within the family, are lymphotropic and include Epstein–Barr virus, human herpesvirus-8 (HHV-8), and Herpesvirus saimiri. The predominant sites of latency of the third subgroup, the β-herpesviruses – cytomegalovirus and human herpesviruses 6 and 7 (HHV-6 and HHV-7) – are not known. 153 Concurrent infection by two viruses of this family is a rare occurrence, seen in immunocompromised patients.154. and 155.
Type-specific identification of the two main types of herpes simplex virus can be made in paraffin sections using immunoperoxidase techniques; furthermore, varicella-zoster virus can be distinguished from them.156. and 157. PCR techniques can also be used.154.158. and 159.
There are two main types of herpes simplex virus, type 1 (HSV-1) and type 2 (HSV-2).151.160. and 161. Primary infection with HSV-1 usually occurs in childhood and is mild. Recurrent lesions occur most commonly around the lips (herpes labialis – ‘cold sores’). Other sites of infection include the oral cavity, pharynx, esophagus, eye, lung, and brain.162.163. and 164. Orofacial herpes is quite common with an annual prevalence of approximately 15%. 165 Its lifetime prevalence is close to 40%. 166 Women are more commonly affected than men. 165 It affects over 40 million people in the United States. 167
Infection with HSV-2 generally involves the genitalia and surrounding areas after puberty; it is usually sexually transmitted.168.169. and 170. Its occurrence in children may be a consequence of child abuse. 171 Approximately 1.6 million persons are infected with HSV-2 annually in the United States. 167 In one study, antibodies to HSV-2 were present in nearly 5% of sexually active Turkish individuals. 172 The incubation period for genital lesions averages 5 days. HSV-2 may also result in generalized or cutaneous lesions of the newborn.173. and 174. The relationship between the site of infection and HSV type is not absolute.175. and 176. Sometimes herpes simplex infection masquerades as some other disease. 177 It may also be difficult to diagnose in immunosuppressed patients. 167 Genital herpes has masqueraded as a cutaneous T-cell lymphoma in two immunosuppressed patients. 178
Once infected, a person will usually harbor the virus for life. The virus can travel along sensory nerves to infect the neurons in the sensory ganglia. Recurrent disease follows this latency in the sensory ganglia and can be stimulated by ultraviolet light, 179 trauma, fever, HIV infection, 180 menstruation, and stress, to name the most common factors. 181 Acute skin eruptions that are positive for HSV DNA polymerase are an increasing problem in patients who undergo stem cell transplantation. 182 No precipitating factors can be identified in some patients. 183 Prostaglandins and diminished production of IFN-γ may play a role in the reactivation of infections.184. and 185. A study some years ago found that reactivation of genital HSV-2 infection in asymptomatic seropositive persons is quite frequent. 186 Asymptomatic perianal shedding of HSV is common in patients with AIDS. 187
The usual lesions of herpes simplex consist of a group of clear vesicles which heal without scarring, except in cases where secondary bacterial infection supervenes. Leukoderma may develop on the lip after herpes labialis. 188 Special clinical variants189 include herpes folliculitis of the beard or scalp, herpetic whitlow190.191.192. and 193. (usually in medical or nursing personnel), necrotizing and ulcerative balanitis194. and 195. and vulvitis196 (a very rare HSV-2 complication, often associated with HIV infection), a varicella-like eruption, 197 infection localized to sites of atopic dermatitis, 198 pulsed-dye laser treatment, 199 or photoexposure, 200 vegetating plaques,201. and 202. hypertrophic papulonodules, 203 flaccid intracorneal blisters, 204 acquired lymphedema of the hand, 205 and eczema herpeticum (see p. 616). 206 Severe primary or secondary infection with systemic involvement may occur in immunocompromised patients.207. and 208. It has followed meningococcal meningitis. 209 Disseminated herpes simplex infection is a rare complication of pregnancy. 210 Recurrent herpes labialis has developed during treatment of acne with isotretinoin.211. and 212. Recurrences decreased after the application of sunscreens. 212 Herpes simplex virus DNA is present in lesional skin in a significant number of patients with erythema multiforme (see p. 51).213. and 214. Lymphedema of the hand is a rare complication of recurrent infection. 215
HSV-1 and HSV-2 are biologically and serologically distinct. They are usually isolated using human embryonic fibroblast cell cultures. 216 Characteristic cytopathic changes can be seen after 1 or 2 days. Rapid diagnosis of cutaneous infections of herpes simplex can be made using smears of lesions and monoclonal antibodies, with an immunofluorescence technique. 217 PCR-based tests are far more sensitive than immunofluorescence. 218 Reliable and convenient serological tests for antibodies against both HSV-1 and HSV-2 are now available commercially. 219 These methods indicate that viral culture has significantly underestimated the number of infected individuals. 219 PCR-based methods are now being used routinely in some laboratories for diagnosis. 220 Confocal scanning laser microscopy has been used at the bedside to diagnose infection with herpesvirus. 221
A range of antiviral agents, topical, oral, and intravenous, have been used in the treatment of herpes simplex, both HSV-1 and HSV-2.222.223. and 224. Topical treatment is the usual method, with oral and intravenous therapy reserved for recurrent and persistent lesions, usually in immunocompromised patients. Docosanol 10% cream is an over-the-counter preparation with indirect antiviral activity approved for topical treatment of herpes simplex labialis. Penciclovir 1% cream and acyclovir (aciclovir) 5% ointment are topical antiviral agents that require a prescription in some, but not all countries. A recent randomized clinical study found that acyclovir cream 5% was equally as effective as Compeed® cold sore patch, based on hydrocolloid technology, in the treatment of herpes labialis. 225 Idoxuridine 15% solution, and cidofovir 1% gel can also be used. 161
Systemic medications used include valacyclovir (valaciclovir) and famciclovir, which has improved oral absorption. 161 Foscarnet and cidofovir can be used intravenously for immunocompromised patients with acyclovir-resistant disease.151. and 161. Acyclovir resistance is uncommon in healthy individuals.151. and 226. In patients with AIDS it is 10%. 226 It has a good safety record and is well tolerated by patients. 151 Isotretinoin has also been used in the treatment of recurrent herpes simplex. 212
A meta-analysis reported in 2007 confirmed the high clinical efficacy of oral acyclovir, valacyclovir, or famciclovir for prophylaxis against recurrent genital herpes infection. 227 Valacyclovir significantly reduces HSV-2 shedding. 228
Experimentally, inhibition of HSV-1 has been produced by a sequence-specific gene-silencing process mediated by small interfering RNA. 229 This is a promising method of gene therapy in the future treatment of a variety of viral diseases.
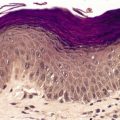
The histological appearances of herpes simplex, varicella, and herpes zoster are very similar. The earliest changes involve the epidermal cell nuclei, which develop peripheral clumping of chromatin and a homogeneous ground-glass appearance, combined with ballooning of the nucleus. 230 Vacuolization is the earliest cytoplasmic alteration. These changes begin focally along the basal layer, but soon involve the entire epidermis. 230 By the time lesions are biopsied there is usually an established intraepidermal vesicle (Fig. 26.9). This results from two types of degenerative change, ballooning degeneration and reticular degeneration. Ballooning degeneration is peculiar to viral vesicles. The affected cells swell and lose their attachment to adjacent cells, thus separating from them (secondary acantholysis). The cytoplasm of these cells becomes homogeneous and intensely eosinophilic, and some are also multinucleate (Tzanck cells). At times the basal layer of the epidermis is also destroyed in this way, leading to the formation of a subepidermal vesicle. The change known as reticular degeneration is characterized by progressive hydropic swelling of epidermal cells, which become large and clear with only fine cytoplasmic strands remaining at the edge of the cells. These eventually rupture, contributing further to the formation of a vesicle. This change is not specific for viral infection and can be seen also in allergic contact dermatitis. Whereas ballooning degeneration is found mainly at the base of the vesicle, reticular degeneration is seen on its superficial aspect and margin.
(A) Herpes simplex. (B) There is an intraepidermal vesicle containing ballooned, acantholytic keratinocytes in which there are intranuclear inclusion bodies. (H & E)
Eosinophilic intranuclear inclusion bodies are found, particularly in ballooned cells (Fig. 26.10). They are more common in multinucleate cells of lesions that have been present for several days. Neutrophils are present within established vesicles. There are also moderate numbers in the underlying dermis, as well as lymphocytes. Neutrophils are prominent in the lesions of herpetic whitlow. Marked inflammation and even vasculitis have been noted in some lesions. 231 As a rule, the dermal inflammation is more severe in herpes simplex than in zoster. Atypical lymphoid cells may be present in the infiltrate where herpes simplex complicates an underlying hematological malignancy. 232 Atypical lymphocytes have been noted in 32 of 45 routine cases of herpes simplex submitted for microscopy. 233 In a study reported from Graz in 2006, atypical lymphocytes were commonly present. 234 Other findings included dense lymphoid infiltrates, angiotropism, and variable numbers of CD30+ and CD56+ cells. Two cases with a pseudolymphomatous appearance revealed a monoclonal population of T lymphocytes by PCR analysis. 234 In another study, CD4+ and/or CD8+ lymphocytes were present in the infiltrate of herpes simplex. 235 The cells expressed both granzyme B and granulysin. 235 In a patient with chronic lymphocytic leukemia, a neoplastic infiltrate of cells occurred at the site of a florid herpes simplex infection, but this seemed to be part of a physiological response to the virus. 236
Herpes simplex. The multinucleate keratinocytes have intranuclear inclusion bodies. (H & E)
Uncommonly, erythema multiforme-like changes may be seen in the adjacent skin, concurrent with a vesicle of herpes simplex. A related reaction pattern is so-called ‘lichenoid lymphocytic vasculitis’, a term coined for the changes seen in presumptive cases of herpes simplex with an immunological response (see p. 608). 237 There is an upper dermal infiltrate of lymphocytes and histiocytes, with lichenoid changes in the epidermis and a dermal lymphocytic vasculitis.
Focal involvement of pilosebaceous units is not uncommon in recurrent lesions. 230 In the variant known as herpes folliculitis, 238 pilosebaceous involvement is the dominant lesion (Fig. 26.11). Rarely, the eccrine ducts and glands are involved (herpetic syringitis). 239 Ballooning degeneration may involve cells of the outer root sheath of the deep portion of the follicle. A dermal inflammatory infiltrate is often present in these cases; it is heaviest in the deep reticular dermis – a so-called ‘bottom-heavy’ infiltrate.
Herpes folliculitis. There is a heavy superficial and deep dermal infiltrate of lymphocytes. A necrotic follicle is present in the upper third of the dermis. (H & E)
Dermal nerves in lesional skin in both herpes simplex and herpes zoster show perineural and some intraneural inflammation. Viral antigen can be detected in these inflamed nerve twigs, indicating that they are not just passive conduits for viral spread. 240 Perineural inflammation can also be present in quiescent lesions of herpes labialis. Schwann cell hypertrophy and neuronal necrosis with cytopathic changes may also be present. 240 Sometimes the perineural infiltration is out of proportion to the overlying dermal inflammation.
In late lesions of herpes simplex, ulceration is often present. Ghosts of acantholytic, multinucleate epithelial cells with slate-gray nuclei on routine staining may still be seen in the overlying crust.
Recently, attention has been drawn to the histological appearance of the lesions that recur at the site of previous surgery. These vesicles are subepidermal, with an inflammatory response, complete with multinucleate giant cells, 241 in the uppermost dermis.
A rapid cytological diagnosis of a vesicular lesion can be made by making a smear from the base of a freshly opened vesicle and staining it with the Giemsa stain. The Tzanck test, as this smear is called, is not as sensitive as PCR-based tests. 242 Ballooned cells, some of which are multinucleate, will be seen in herpes simplex, varicella, and zoster. Immunoperoxidase stains specific for HSV-1, HSV-2, and VZV are available commercially.
Virus particles of Herpesvirus hominis, measuring between 90 and 130 nm, can be seen in the nuclei of the basal cells. Cells in the malpighian layer often contain other virus-related material, such as nuclear granules and capsules. Viral capsids have also been seen within the nuclei of monocytes, young histiocytes, and lymphocytes in the epidermal vesicles. 243 Large lymphocytes are sometimes seen adjacent to keratinocytes exhibiting lytic changes, suggesting that cell-mediated immunity may be partly responsible for the epidermal damage that occurs. 244
Eczema herpeticum is a generalized infection of the skin with the herpes simplex virus.245.246. and 247. A similar condition (eczema vaccinatum) occurred, in the past, with the vaccinia virus. 248 Both lesions have been grouped together as Kaposi’s varicelliform eruption. This condition occurs most commonly in association with atopic dermatitis, 249 but it has also been reported in Darier’s disease,250. and 251. Hailey–Hailey disease, 252 Grover’s disease, 253 pityriasis rubra pilaris, 254 allergic contact dermatitis, 255 pemphigus foliaceus, seborrheic dermatitis, rosacea, 256 psoriasis,257. and 258. lupus vulgaris, 257 ichthyosiform erythroderma, phenytoin-induced drug rash, 259 a patient receiving facial tacrolimus treatment for atopic dermatitis, 260 and in multiple myeloma, 261 the Sézary syndrome and mycosis fungoides, 262 and following thermal injury. 263 It has also been reported in an HIV-positive patient after laser resurfacing. 264 It has been suggested that interleukin-4, which may be increased in atopic dermatitis, may down-regulate the response against herpes simplex virus and contribute to generalized infection. 265 Reduced numbers of natural killer (NK) cells and a decrease in interleukin-2 receptors probably also contribute. 266
Although eczema herpeticum is characterized by the presence of multinucleate epidermal cells and intranuclear inclusions, rather than the intracytoplasmic inclusions seen in the past with eczema vaccinatum, these features are often obscured by the heavy inflammatory cell infiltrate of neutrophils and early breakdown of the vesicles. Recently formed lesions usually show the typical features of a vesicle of herpes simplex.
Varicella (chickenpox), caused by the varicella-zoster virus (VZV), is a highly contagious disease with an average incubation period of 2 weeks. 267 It is predominantly a disease of childhood. The peak incidence of infection is between 5 and 9 years of age. 268 It is characterized by an acute vesicular eruption; bullous lesions are rare.269. and 270. Postinflammatory scarring of isolated lesions is very common, with a prevalence of just under 20%. 268 The lesions develop in successive crops, so that the rash typically consists of pocks at different stages of development. Thus, papules, vesicles, pustules, crusted lesions, and healing lesions may all be present. Secondary Staphylococcus aureus infections are widely reported. 269 Varicella pneumonia may be seen in adults as a primary infection. Other complications of chickenpox include thrombocytopenia, necrotizing fasciitis, cerebellar ataxia, encephalitis, aseptic meningitis, Guillain–Barré syndrome, Reye’s syndrome, Henoch–Schönlein purpura, and orchitis. 268 In immunocompromised hosts dissemination may occur, or large ulcerated and necrotic lesions (varicella gangrenosa) may develop; chronic verrucous lesions have also been described.271.272. and 273. Atypical recurrent varicella also occurs in patients with hemopathies, 274 and those receiving hemodialysis. 275 An interesting observation is the finding of marked intensification of the varicella eruption in areas of skin which are normally covered but which become sunburnt just before the eruption commences. 276 Photodistributed varicella, mimicking polymorphic light eruption, has been reported. 277 Involvement of the palms and soles is uncommon. 278 Varicella has also been reported largely restricted to an area covered by a plaster cast, 279 and in a patient with Kawasaki disease. 280 It has developed in a patient receiving adalimumab therapy. 281 Viral exanthems, particularly varicella, may localize early and preferentially to areas of prior inflammation. 282
Cutaneous lesions, usually scars, can be seen in neonates with the congenital varicella syndrome. This is a rare syndrome which occurs when a pregnant woman develops varicella before the 24th week of pregnancy. In addition to scars, aplasia cutis, neurological defects, eye abnormalities, and limb hypoplasia may be seen, but less frequently. 283 The incidence of an embryopathy after infection in the first 24 weeks of gestation is estimated at 1–2%. 284
Usually the diagnosis of varicella is made on clinical grounds. In atypical cases, PCR for VZV can be performed. 281
Immunization against the varicella-zoster virus can be carried out using the Oka strain of live, attenuated varicella. Care should be taken to ensure that recipients are not immunocompromised when receiving live vaccines; varicella may result. 285 While the overall benefit of treating uncomplicated cases of varicella in otherwise healthy children has not been considered significant by some clinicians, treatment with oral acyclovir, within 24 hours of the development of the rash, is cost-effective because it allows caregivers to return to work approximately 2 days earlier. 224 Acyclovir does not appear to alter the development of long-term immunity to VZV. 224 The newer antiviral agents have not been extensively studied.
The appearances are virtually indistinguishable from those of herpes simplex, although the degree of inflammation is said to be greater in herpes simplex than in varicella-zoster lesions. 286 Direct immunofluorescence and immunoperoxidase methods, 156 using a monoclonal antibody specific for varicella-zoster virus, 287 and the Tzanck smear are superior to viral culture in the diagnosis of varicella zoster; 288 monoclonal antibody techniques have the advantage of specificity over the Tzanck smear. 287
The chronic verrucous lesions reported in immunocompromised patients show pseudoepitheliomatous hyperplasia and massive hyperkeratosis. 271 Herpetic cytopathic changes are also present in keratinocytes.
The ultrastructural features of Herpesvirus varicellae are similar to those of Herpesvirus hominis. However, colloidal gold immunoelectron microscopy using monoclonal antibodies can distinguish between the two. 289
Herpes zoster (shingles) is a common dermatological condition that affects 10–20% of the population during their lifetime, with an increased incidence in the elderly, and in those who are immunocompromised. 290 An estimated 1 million cases of herpes zoster occur each year in the United States. 291 It results from reactivation of latent varicella-zoster virus (VZV) infection. The characteristic rash has a unilateral dermatomal distribution that most often affects the thoracic and lumbar regions, and sometimes the face. Any dermatome can be affected. Rare localizations have included involvement of the penis, 290 the finger, 292 and a recent surgical scar. 293
Herpes zoster is an acute disease that occurs almost exclusively in adults. Childhood herpes zoster is uncommon and usually restricted to immunocompromised children, and those with malignancies.294. and 295. It can develop in immunocompetent children as a consequence of intrauterine VZV infection, or postnatal exposure to this virus at an early age. 296 The illness is febrile and begins with pain in the area innervated by the affected sensory ganglia. The skin in this area becomes red, and papules soon develop. 297 These quickly transform into vesicles and then pustules. There is one report of bullous lesions. 298 Crusts then form and, later, healing takes place. Chronic hyperkeratotic and verrucous lesions may occur in immunocompromised patients.299.300. and 301. Verrucous lesions have been reported in an immunocompetent child who developed chronic varicella-zoster skin infection complicating the congenital varicella syndrome. 283 Chronicity appears to be associated with a particular pattern of viral gene expression with reduced or undetectable levels of the viral envelope glycoproteins gE and gB. 300 Sometimes there is residual scarring, particularly if there has been secondary bacterial infection of the vesicles. Although the virus usually causes a prodrome of pain, pruritus, or a burning sensation, uncommon presentations have included hiccups, 302 eructation, the Ramsay Hunt syndrome, 303 urinary and fecal retention, and sexual dysfunction. 302
Herpes zoster is caused by the same virus as varicella (VZV).304.305. and 306. It occurs in individuals with partial immunity resulting from a prior varicella infection. With few exceptions, zoster appears to represent reactivation of latent virus in sensory ganglia, often in an immunocompromised host. There appears to be an association between herpes zoster and a family history of zoster. 307 The risk increases when multiple blood relatives have been involved. 307 The therapeutic use of arsenic trioxide can lead to reactivation of the VZV. 308 The virus travels through the sensory nerves to reach the skin, where it replicates in the epidermal keratinocytes. Viremia may occur but it is not consistent. 309 Now that a vaccine is available, it is hoped that this may lessen the incidence of herpes zoster in the future. 310 Nevertheless, herpes zoster has been reported in a child following immunization for varicella using the Oka strain of live, attenuated varicella. This strain was recovered from lesional skin. 311
Zoster is not highly infectious, although this has been recently disputed. 312 When children are infected by adults suffering from zoster they develop varicella and not zoster. An attack of either disease leaves the patient with some measure of immunity against both. Recurrent attacks of zoster are most uncommon. 304 Many cases diagnosed as recurrent herpes zoster are probably recurrent herpes simplex.313. and 314. Herpes simplex virus has been detected by PCR in two cases with a persistent, painful papular eruption in a zosteriform pattern. 315 Conversely, there is also evidence from PCR studies that initial herpes zoster is sometimes misdiagnosed as herpes simplex. 316 Simultaneous varicella zoster and herpes simplex infection also occurs, even in immunocompetent individuals. 317 PCR is the method of choice for the early diagnosis of herpes zoster. 318 Disseminated herpes zoster with visceral involvement is a rare complication of AIDS and other immunocompromised states.306.319.320. and 321. It has also been reported in idiopathic CD4+ lymphocytopenia. 322
Herpes zoster scars are prone to the occurrence of Wolf’s isotopic response, which describes the development of a new skin disorder at the site of another unrelated and already healed skin disease. The diseases reported in healing/healed lesions of herpes zoster include granuloma annulare, 323 granulomatous dermatitis, 324 comedones,325. and 326. lichen planus, 327 giant cell lichenoid dermatitis, 328 urticaria, 329 granulomatous vasculitis, granulomatous folliculitis, rosacea, 330 sarcoidosis, 331 lichen sclerosus et atrophicus, morphea, an eosinophilic dermatosis, fungal infections, 332 pseudolymphoma, 333 lymphoma, 334 leukemia cutis, 335 Rosai–Dorfman disease, Kaposi’s sarcoma, various skin cancers, and metastases.336. and 337. Necrotizing fasciitis is another rare complication of disseminated cutaneous herpes zoster. 338 VZV has been inconsistently identified in some of these post-herpetic inflammatory reactions. Virus was identified in a patient with cutaneous lymphoid hyperplasia and concomitant folliculitis and vasculitis. 339
Post-herpetic neuralgia is a serious complication. 340 It can cause debilitating pain and impaired quality of life. 291 Rash severity appears to correlate with prolonged post-herpetic neuralgia; 341 it is also more prevalent in patients over 50 years of age. 342 Severe ocular damage may result when the ophthalmic division of a trigeminal nerve is involved. 343 If multiple dermatomes are involved, they are usually contiguous. There is one report of an immunocompromised patient in whom seven disparate dermatomes were involved (zoster multiplex). 344
The host’s immune response to viral skin infections is a complex one combining the effects of natural killer (NK) cells, interferons (IFN), and macrophages which restrict viral replication and spread. 345 Both CD4+ and CD8+ T cells are also involved. For their part, the viruses produce substances that allow them to escape, in part, the host’s immune recognition. 345 This is a complex process involving down-regulation of both MHC-I and MHC-II pathways. Intercellular adhesion molecule-1 (ICAM-1) expression on the surface of keratinocytes allows binding to occur with a certain class of T cells (LFA-1 ligand-bearing T cells) involved in the host’s response to viruses. It has now been found that ICAM-1 expression is lost on VZV-infected keratinocytes, reducing their capacity to bind with these T cells, suggesting a further immune-evasion strategy by the virus. 345
The treatment of herpes zoster involves the use of antiviral drugs, or combination regimens using these drugs with corticosteroids to reduce the severity and/or duration of the disease, and the use of other strategies to decrease the pain of any post-herpetic neuralgia that may develop. 346 In this latter category are opioids, anticonvulsants such as gabapentin, tricyclic antidepressants, capsaicin cream, and lidocaine patches 5%.
Acyclovir had been the drug of choice for many years, but now the newer drugs famciclovir and valacyclovir are preferred because of their superior pharmacokinetic characteristics and simpler dosing regimens. 342 Antiviral therapy should be commenced within 72 hours of the onset of the rash, although therapy initiated after this time appears to be still helpful. In a meta-analysis involving 691 patients, acyclovir was found to reduce zoster-related pain duration and prevalence by about half. 342 Similar studies with the newer antiviral agents have not yet appeared. PCR can be used to detect mutations or deletions in the thymidine kinase gene in herpes simplex and VZV which confers on the viruses a resistance to acyclovir. 347
Administration of gabapentin in conjunction with antivirals during the acute phase of zoster may offer further protection against post-herpetic neuralgia. 342 The addition of corticosteroid may, in the short term, reduce herpes-related pain intensity, but it is associated with a risk of serious adverse effects. Antiviral therapy is essential in patients presenting with herpes zoster ophthalmicus, and in patients who are immunocompromised. 342
The prevention of herpes zoster involves the use of vaccination. In 2006, the US Food and Drug Administration (FDA) approved the use of a live attenuated preparation of the Oka/Merck strain of VZV for clinical use. Its potency is said to be 14 times that of the varicella vaccine that preceded it.348. and 349. It boosts the recipient’s immunity to VZV, reducing the chance that the individual’s latent VZV will reactivate producing zoster. 346 It reduces the incidence of zoster by 50%, and in those who still contract the disease, it reduces the rate of post-herpetic neuralgia by 66%. 348 Its cost will be of concern in some countries.
The appearances resemble those described for herpes simplex. Despite the comment made earlier that lesions of herpes simplex are usually more inflammatory than those due to varicella-zoster infection, 286 dermal inflammation may be prominent in some cases of herpes zoster, and there may occasionally be a vasculitis. 350 If the vasculitis is severe, necrotizing lesions will be present. 351 A granulomatous vasculitis and lesions resembling granuloma annulare may be seen in healing or healed lesions.323.352. and 353. These factors, as well as secondary infection, contribute to the scarring which sometimes ensues.
Eccrine duct and secretory coil involvement has been reported, but it is quite uncommon. 354 Concomitant epidermal involvement is not always present in these cases. 355 Folliculosebaceous involvement is more common. It is thought that exclusive folliculosebaceous involvement, in the setting of a non-vesicular eruption (that is, without initial epidermal involvement) represents early herpes zoster. 356 This is probably a consequence of the virus traveling via myelinated nerves to the skin that terminate at the isthmus of the hair follicles, in contrast to recurrent herpes simplex in which transport of the virus is to the epidermis, via terminal non-myelinated nerve twigs. 356
The chronic verrucous lesions show hyperkeratosis, verruciform acanthosis, and virus-induced cytopathic changes. 300
The term ‘herpes incognito’ has been used for the cases in which the typical multinucleated cells are not encountered in routine sections. 357 Such cases usually show a lichenoid lymphocytic vasculitis, often with subepidermal edema, and often with a periadnexal lymphocytic infiltrate with variable sebaceitis. 357 Deeper levels in such cases will often show more typical features.
Cytomegalovirus (CMV) belongs to the subgroup of β-herpesviruses. Like other members of the family Herpesviridae, this virus produces primary infection, latent infection, and reinfection; 358 however, its site of latency is not known.
There are few reports of cutaneous involvement with CMV.359.360. and 361. A maculopapular eruption is the most common clinical presentation, and is seen most often in patients with CMV infection who are treated with ampicillin. This is analogous to the situation in infectious mononucleosis. 360 Urticaria, 362 vesiculobullous lesions, 363 pustular lesions, 364 ulceration,365. and 366. including genital ulcers, 367 keratotic lesions, 368 diaper dermatitis, 369 and even epidermolysis have been reported. Cytomegalovirus was demonstrated in the dermis in a patient with pityriasis lichenoides (PLEVA). 370 Its etiological role was uncertain. Magro et al have reported seven adults who developed a cutaneous vasculopathy or sclerodermoid changes in temporal association with recent CMV infection. 371 No CMV inclusion bodies were present. 371 Often the clinical picture is quite non-specific.372. and 373. The patients are usually immunocompromised,363.374. and 375. a clinical setting in which mixed infections with other agents, particularly herpes simplex virus, may occur.358.376.377.378.379.380. and 381. Patients with chronic renal failure are also prone to infection. 382
Infants with congenital infection with CMV may present with petechiae and blue-red macular or plaque-like (so-called ‘blueberry muffin’) lesions, in addition to neurological abnormalities. 383 Perineal ulcers were present in one infant. 384 The lesions resolved without specific therapy.
Ganciclovir, a guanosine analogue that selectively inhibits CMV DNA polymerase, can be used to treat infections. 384 Foscarnet and valganciclovir have also been used. 385 There is still a high mortality in patients with disseminated disease, but when there is local cutaneous infection in skin wounds, the prognosis is usually good. 382
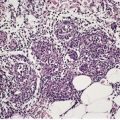
There is usually a non-specific dermal infiltrate. The characteristic changes are enlarged endothelial cells in small dermal vessels (Fig. 26.12). 360 The nuclei of these cells contain large eosinophilic inclusions, surrounded by a clear halo. Cytomegalic changes in the absence of nuclear inclusions have been reported. 386 There may also be prominent neutrophilic infiltration of the involved vessel walls, although an unequivocal leukocytoclastic vasculitis is quite rare. 387 Other cells that may harbor viral inclusions include fibrocytes and macrophages. Ductal epithelial cells are rarely involved. 388 The ‘blueberry muffin’ lesions associated with congenital infections are the result of dermal erythropoiesis. 383 They may also be seen in congenital rubella infections. 383
Cytomegalovirus infection. A blood vessel in the dermis contains several enlarged endothelial cells, each containing an inclusion body. (H & E) (Photomicrograph kindly supplied by Dr G Strutton)
Epstein–Barr virus (EBV) was discovered in 1964 in African Burkitt lymphoma-cell cultures. 391 It belongs to the human γ-herpesvirus subfamily along with human herpesvirus-8 (HHV-8). It is best known as the causative agent of infectious mononucleosis, following which it establishes a clinically silent lifelong infection. It also causes a range of natural killer (NK)-cell malignancies as well as B- and T-cell lymphomas, and a hemophagocytic lymphohistiocytosis (see p. 985), which is often fatal. Carcinomas and leiomyosarcomas may also result from EBV infection. Many of these tumors are found predominantly in southeast Asia and Japan. They were reviewed recently.391. and 392.
A cutaneous rash is seen in approximately 10% of patients with infectious mononucleosis, but this incidence increases dramatically if ampicillin is administered to the patient. It also occurs when ampicillin is used in patients with EBV reactivation. 393 The rash is usually erythematous, macular, or maculopapular. Erythema multiforme and urticaria may occur. 1 Rare cutaneous manifestations of EBV infection include a granuloma annulare-like eruption, 394 the Gianotti–Crosti syndrome, 395 painful genital ulcers,396. and 397. oral hairy leukoplakia, and lymphoproliferative lesions in immunocompromised patients, particularly following transplantation. 398 Although the lesions may histologically mimic malignant lymphoma, the lesions disappear completely in some patients after the degree of immunosuppression is lowered.398.399. and 400. Epstein–Barr virus has also been implicated in the etiology of Kikuchi’s disease,401. and 402. and in a natural killer (NK) cell lymphocytosis associated with hypersensitivity to mosquito bites. 403
Extreme sensitivity to infection with EBV is seen in X-linked lymphoproliferative syndrome (XLP1 – OMIM 308240), caused by a mutation in the SHD2D1A gene encoding SLAM-associated protein located at Xq25.404. and 405. A small number of cases (XLP2 – OMIM 300635) are caused by mutations in the gene encoding the X-linked inhibitor of apoptosis (XIAP/BIRC4), which maps to the same region as the gene for XLP1. 404 Both diseases have a similar phenotype with acquired hypogammaglobulinemia and a malignant lymphohistiocytosis. Early transplantation of allogeneic hematopoietic stem cells is the only means available to prevent fatal EBV complications later in life. Similar cases, in which no genetic abnormality has been detected, are sometimes seen. 406
In-situ hybridization for EBV-encoded small nuclear RNA (EBER) remains the gold standard for virus detection in tissue samples. 391
Both oral and intravenous acyclovir have shown little or no clinical benefit in the treatment of uncomplicated infectious mononucleosis. 224 Acyclovir, ganciclovir, penciclovir, famciclovir, foscarnet, zidovudine, and interferon have all been shown to have in-vitro efficacy against the replication of EBV. 224
There is usually a mild perivascular infiltrate of inflammatory cells. The changes are non-specific.
Human herpesvirus-6 (HHV-6) is the sixth member of the family Herpesviridae to be identified. It shows closest homology with cytomegalovirus and HHV-7. 407 It is a member of the β-herpesvirus subfamily. Most of the reports of this infection are in the pediatric literature. The virus produces a cutaneous eruption (exanthem subitum) resembling measles or rubella in infants. 408 The illness may be accompanied by fever. Like other herpesviruses, after the primary infection, it establishes latency in different cells and organs. 409
HHV-6 infection in infants is said to be the commonest cause of fever-induced seizures.407. and 410. In adults, infection is seen primarily in immunocompromised individuals. Its reactivation may be involved in the pathogenesis of the rash and GVHD that may follow allogeneic stem cell transplantation. 411 Herpes simplex virus (HSV) may also be reactivated in these circumstances. 182 Although HHV-6 may play a role in multiple sclerosis and a demyelinating disease in patients with HIV infection, it plays no role in the etiology of lymphomatoid papulosis. 410 Although parvovirus B19 is usually implicated in the etiology of papular-purpuric ‘gloves-and-socks’ syndrome (see p. 626), a case has been reported in association with HHV-6 infection. 412 The Gianotti–Crosti syndrome may also be produced by HHV-6 infection. 413
There are now many reports of a drug hypersensitivity syndrome occurring in association with reactivation of HHV-6.414.415. and 416. This has been called the drug-induced hypersensitivity syndrome (DIHS) or drug rash with eosinophilia and systemic symptoms (DRESS). 417 It is considered further in Chapter 20, p. 515. In one case a fulminant hemophagocytic syndrome developed418 and in another toxic epidermal necrolysis. 409 HHV-7 may also act in concert with HHV-6 in producing the drug hypersensitivity syndrome. 415 CMV and/or EBV have also been implicated. 419 Their reactivation occurs in a similar sequential order to that seen in GVHD. 420 The drugs initially implicated were sulfasalazine, 414 allopurinol, 415 and phenobarbital. 418 Many other drugs have since been implicated (see p. 515). There is some controversy regarding the role of HHV-6 and HHV-7 in the etiology of pityriasis rosea (see p. 100). 421 It is possible that viral reactivation is the explanation for its detection in a number of circumstances. 422
The cutaneous lesions of exanthem subitum are characterized by spongiosis, small spongiotic vesicles and exocytosis of lymphocytes, sometimes producing Pautrier-like lesions. There is often edema of the papillary dermis and a superficial perivascular infiltrate of mononuclear cells. 408 Cytopathic changes, resembling those seen in herpes simplex and varicella-zoster infections, are absent.
Human herpesvirus-7 (HHV-7) was first isolated from a peripheral blood T cell in 1990, and isolated again in 1992 from a patient with chronic fatigue syndrome. 410 HHV-7 can provide a transactivating function for HHV-6. HHV-7 is ubiquitous and infects more than 80% of children in infancy. 410 It has been implicated in the etiology of pityriasis rosea (see p. 100), but reactivation of the virus is a possible explanation for its occurrence in some cases. There is no clear link between HHV-7 and any specific disease at this time. 423
Unlike the other recently described herpesviruses (HHV-6 and HHV-7), this virus (HHV-8) does not appear to be ubiquitous. 424 Its role in the etiology of Kaposi’s sarcoma in HIV-positive individuals was confirmed in 1994 when unique DNA sequences were isolated from biopsies of Kaposi’s sarcoma. The virus, initially called Kaposi’s sarcoma-associated herpesvirus (KSHV), was subsequently renamed HHV-8. Sequence analysis confirms that it is related to Herpesvirus saimiri, which induces lymphoid malignancies in some primates, and to Epstein–Barr virus (EBV). 425 It is a member of the γ-herpesvirus subfamily along with these two viruses. 426
The role of HHV-8 in the etiology of Kaposi’s sarcoma in patients infected with HIV is now beyond doubt.427.428. and 429. It has also been found in a variable but significant number of HIV-negative cases. 430 The viral load of HHV-8 is relatively low in both HIV-positive and HIV-negative cases. 431 HHV-8 infects the endothelial-derived spindle cells of Kaposi’s sarcoma as well as CD19+ B cells. This latter event may be etiologically significant in the causation of some cases of Castleman’s disease (see p. 946) and primary effusion lymphoma. 432 It has also been found in lymphomas and in other lymphoproliferative disorders with heterogeneous presentations. 433 This subject has been reviewed recently.432. and 433. Its presence in a surprising number of skin cancers, and in lesions of pemphigus has not been satisfactorily explained, although tropism for lesional skin has been postulated.434.435. and 436. Other studies have failed to confirm these findings.437. and 438. It is not associated with pityriasis rosea. 439
Papovaviruses (papillomaviruses) are DNA viruses which replicate in the nucleus. The only important virus in this group in dermatopathology is the human papillomavirus (HPV), which produces various types of warts on different parts of the skin.441.442.443.444. and 445. The use of new techniques, such as DNA hybridization, has allowed the separation of over 120 antigenically distinct strains of HPV.446. and 447. Further genotypes have been identified, but not fully characterized. 448 PCR is now used routinely for the typing of HPV. 347 In recent years attempts have been made to relate specific antigenic strains of HPV to particular clinicopathological groups of verrucae.449. and 450. Some strains have oncogenic potential, and the theoretical mechanisms by which HPV may cause cancer have been reviewed, 451 as has the role of the various immune defense mechanisms to these oncogenic and other strains of HPV.452. and 453. The following correlations have been recorded: HPV-1 – plantar warts,454. and 455. but also common warts and anogenital warts; HPV-2 – common warts, but also plantar, 454 oral, 456 and anogenital lesions; HPV-3 – plane warts and epidermodysplasia verruciformis; HPV-3 (variant) – common warts; 457 HPV-4 – plantar and common warts; HPV-5 – epidermodysplasia, which in cases associated with this strain of HPV is usually complicated by carcinoma; HPV-6 – anogenital warts and also epidermodysplasia; HPV-7 – warts in meat and fish handlers;458.459.460.461. and 462. HPV-8, 9, 10, 12, 14, 17, 19, 22, 24, and many others – epidermodysplasia; HPV-11 – anogenital lesions; 463 HPV-13 and 32 – focal epithelial hyperplasia (Heck’s disease);464. and 465. HPV-57 – plantar epidermoid cysts and nail dystrophy, cutaneous verrucae;466.467. and 468. HPV-60 – plantar warts and epidermal cysts, and papular and nodular lesions on the extremities;469. and 470. HPV-63, 65, and 66 – plantar warts; 471 and HPV-75, 76, and 77 – common warts in immunosuppressed patients. 448 HPV-63 appears to have an eccrine-centered distribution in plantar skin. 471 There is now great interest in the role of HPV-16 and 18 in the etiology of uterine cervical intraepithelial neoplasia (CIN) and invasive carcinoma of the cervix. HPV-16 is also the strain most frequently implicated in the etiology of bowenoid papulosis, a disease which sometimes progresses to invasive carcinoma. HPV-16 has also been detected in anogenital warts, 472 and in a squamous cell carcinoma of the finger. 473 Various HPV strains may be present in immunosuppressed patients474 and in skin tumors removed from them.475.476.477. and 478. They may also be found in the skin tumors of immunocompetent individuals. 479 Individuals whose immunosuppression results from HIV infection have an increased prevalence of HPV infections, a more rapid progression of the disease, and a higher number of invasive carcinomas. 480 Recent studies have detected HPV strains usually associated with epidermodysplasia verruciformis in some of the malignant and premalignant skin lesions of renal transplant recipients.481. and 482. This was not found in an earlier study. 483 Furthermore, HPV-5 has recently been found in psoriatic skin lesions, but it is not known if it has any etiological role (see below).
The traditional classification of verrucae will be used here:
• verruca vulgaris or common wart
• palmoplantar warts, including superficial and deep types
• verruca plana
• epidermodysplasia verruciformis
• condyloma acuminatum.
It should be noted that there is some clinical and histological overlap between these groups. 484 Focal epithelial hyperplasia and bowenoid papulosis will also be discussed.
The common wart (verruca vulgaris) occurs predominantly in children and adolescents, although adults are also frequently infected. 485 Warts have been found in approximately 20% of school students. 486 The lesions may be solitary or multiple, and they are usually found on exposed parts, most frequently on the fingers. 487 Uncommonly, verruca vulgaris occurs on covered areas, such as axillae, groins, or genital areas. 488 They are hard, rough-surfaced papules that range in diameter from about 0.2 cm to as much as 2 cm. New warts may form at sites of trauma (Koebner phenomenon), though not so frequently as in cases of plane warts. They are preferentially associated with HPV-2, but may be induced by HPV-1, HPV-4, HPV-27, and uncommonly, HPV-7.464. and 489. HPV-57 has been reported to produce multiple, sometimes recalcitrant cutaneous verrucae; 468 it is not uncommon in patients with HIV infection. 490 In children, HPV-6 and/or 11 are rarely found in common warts, 491 while HPV-75, 76, and 77 have been identified in lesions from immunosuppressed patients of all ages. 448 Disseminated HPV-11 infection has also been reported in a patient with pemphigus vulgaris receiving various treatments. 492 Extensive verrucae have been reported in immunodeficiency syndromes, including CD4+ T-cell lymphocytopenia.493.494.495. and 496. They may clear following the use of highly active antiretroviral therapy (HAART).497. and 498.
While the great majority of genital warts in adults are due to HPV-6 and HPV-11, this is not so in young girls in whom the occurrence of these strains raises the question of sexual abuse. In one study of 29 genital warts in girls less than 5 years of age, 41% were due to HPV-2 and the remainder to HPV-6/11. 499 Lesions positive for HPV-2 often show the marked hyperkeratosis typical of verruca vulgaris in other sites. 499
An interesting observation of uncertain significance is the finding that the antimicrobial peptide LL-37 is expressed by keratinocytes in verruca vulgaris and also condyloma acuminatum. 500
While spontaneous regression of individual warts is quite common, patients are not necessarily wart-free at follow-up, 501 as multiple recurrences are common. This may lead to personal embarrassment, particularly in those playing contact sports. 502 Various therapies have been used including electrocautery, surgical excision, laser therapy, 503 cryotherapy, double-freeze cryotherapy, duct tape with or without salicylic acid beneath this occlusive product, and other keratolytic agents. Over-the-counter salicylic acid is the most cost-effective treatment. 504 A recent (2007) double-blind randomized controlled trial using duct tape in adults found no statistically significant difference between duct tape and the control in the clearance of warts. 505 Other topical therapies have included cantharidin, trichloroacetic acid, imiquimod 5% cream, ketoconazole, tretinoin cream, and podofilox.506.507. and 508. Extensive and recalcitrant lesions have also been treated with oral acitretin. Destructive methods sometimes cause pain and morbidity. 506 Warts have also been successfully managed by homeopathy and by hypnosis. 503 The smoke of burnt leaves of the tree Populus euphratica may be as effective as cryotherapy for the treatment of hand and foot warts. 509 It has long been used in rural Iran for the treatment of warts.
Common warts show marked hyperkeratosis and acanthosis. There is often some inward turning of the elongated rete ridges at the edge of the lesion (Fig. 26.13). There is some papillomatosis, but this is particularly prominent in filiform variants (Fig. 26.14). Columns of parakeratosis overlie the papillomatous projections. Sometimes there is a small amount of hemorrhage within these columns. The granular layer is lacking in these areas, but elsewhere it is thickened, the cells containing coarse clumps of keratohyaline granules (hypergranulosis). A characteristic feature is the presence of large vacuolated cells in the more superficial parts of the malpighian layer and in the granular layer. These cells (koilocytes) have a small pyknotic nucleus surrounded by clear cytoplasm. There may be small amounts of keratohyaline material in the cytoplasm. These vacuolated cells are not seen in older lesions. Warts in renal transplant recipients express keratin 13 (K13) at a much higher rate than warts from immunocompetent individuals. 511
(A) Verruca vulgaris. (B) Note the papillomatosis, large keratohyaline granules, and the characteristic inturning of the rete pegs. (H & E)
(A) Verruca vulgaris of filiform type. (B) There is marked papillomatosis. (H & E)
Cells with pale cytoplasm, representing tricholemmal differentiation, may be seen in the lower epidermis and follicular infundibula of old warts. This has led to controversy regarding the specificity of the appendageal tumor known as tricholemmoma (see p. 767). 512 It seems likely that tricholemmoma is a specific entity, but that local areas of similar appearance can be seen in old warts, particularly if they involve the follicular infundibulum. 513 A similar controversy surrounds the presence of squamous eddies in old warts, and the relationship of these lesions to inverted follicular keratosis (see p. 765). 512 Sebaceous differentiation may rarely be seen. Acantholytic dyskeratosis has been reported in the viral warts that developed in a boy with Darier’s disease. 514
The nuclei of some of the cells in verruca vulgaris may also be vacuolated. They may contain basophilic inclusions representing viral inclusion bodies, or eosinophilic material which is possibly nuclear debris. Papillomavirus antigen can be detected by immunoperoxidase methods. 515
Most warts show few changes in the dermis, although dilated vessels often extend into the core of the papillomatous projections. A review of 500 verrucae at the Mayo Clinic showed that 8% had a lymphocytic dermal infiltrate, with lichenoid features. 516 The patients were usually elderly. The authors suggested that this might represent an immunological response, although there was no clinical evidence of regression. Rarely, a dense inflammatory cell infiltrate is present in the dermis. Up to 10% or more of these cells may be CD30+. 517 Earlier observations suggested that the regression of common warts followed the lesions turning black, a result of thromboses in the capillaries and venules in the upper dermis, accompanied by hemorrhage. However, another study found that in all but one instance, regression took place without the lesions turning black. 518 These verrucae had a heavy mononuclear cell infiltrate in the dermis, with features of a lichenoid tissue reaction. Civatte bodies were not confined to the basal layer. The findings suggest that cell-mediated regression may occur in common warts, similar to that described in plane warts. Warts treated with injections of bleomycin show confluent epidermal necrosis, single apoptotic keratinocytes, and diffuse neutrophil infiltration of the epidermis, with abscess formation in the granular layer. 519
Carcinoma in situ520. and 521. and squamous cell carcinoma have developed only very rarely in common warts.494.512. and 522. Some of the squamous cell carcinomas that develop in renal transplant patients arise in warts. In one study of renal allograft recipients only 6% of warts removed from these patients showed dysplastic (atypical) changes. 523 Multiple, huge cutaneous horns have been reported overlying a verruca vulgaris induced by HPV-2. 524
Wart virus particles range from 45 to 55 nm in diameter. They are initially seen in the nucleus.
Palmoplantar warts are found on the palm of the hand or sole of the foot, and are of clinical importance because they are often painful. They usually occur beneath pressure points, where they may be confused with callosities. They are preferentially associated with HPV-1 or HPV-4 infection, 464 although other subtypes, including HPV-45, 57, 60, 63, 65, and 66, have recently been incriminated (see below). 525 Warts on the palms and soles have traditionally been divided into superficial (mosaic) warts, which are ordinary verrucae, and a deep variety (myrmecia – so named for its supposed resemblance to an anthill). Several other variants469 have recently been described:
2. A pigmented verrucous variant associated with HPV-65.
4. A large plantar wart caused by HPV-66. 531
There are many similarities between the usual variant caused by HPV-1 and the common wart, except that the greater part of the lesion lies deep to the plane of the skin surface and intrudes well into the dermis. 510 There is prominent hyperkeratosis. The HPV-60-associated lesions show acanthosis, but only mild papillomatosis. 532
It is now accepted that there are correlations between certain HPV types and specific cytopathic changes:469. and 530.
• HPV-1 – vacuolated cells in the upper epidermis with large eosinophilic keratohyaline granules (granular inclusions)
• HPV-2 – condensed heterogeneous keratohyaline granules
• HPV-4 – large vacuolated keratinocytes with almost no keratohyaline granules and small peripherally located nuclei
• HPV-60 and HPV-65 – eosinophilic, homogeneous and solitary inclusions, sometimes seen also with HPV-4 (homogeneous inclusions)
• HPV-63 – intracytoplasmic, heavily stained keratohyaline material with filamentous structures that may encase the vacuolated nucleus (filamentous inclusions). Similar inclusions have recently been reported with an HPV genotype that could not be characterized, but which was not HPV-63.533. and 534.
Regression of plantar warts is usually associated with thrombosis of superficial vessels, hemorrhage and necrosis of the epidermis. There is often a mixed inflammatory cell infiltrate. 535
Pigmented warts are associated with one of the related types of HPV (HPV-4, 60, and 65). The pigmentation is due to the presence of ‘melanin blockade melanocytes’, resulting from a failure of the transfer of melanosomes to keratinocytes. 536 As a consequence melanocytes become highly dendritic and engorged with melanin pigment. 536
Plane warts (verrucae planae) are flesh-colored or brownish, flat-topped papules, a few millimeters in diameter. They occur most frequently on the back of the hands and on the face. The warts are preferentially associated with HPV-3 and HPV-10; 464 HPV-5 is rarely involved in patients with HIV infection.537. and 538. Multiple plane warts have been reported in a patient with idiopathic CD4+ T-cell lymphocytopenia. 539 They may develop at sites of trauma (the Koebner phenomenon). Plane warts may disappear suddenly after a few weeks or months, or they may persist for years. Involution is usually preceded by an erythematous change in the warts, 540 but other presentations have included the development of depigmented haloes, 541 and the sudden eruption of large numbers of tiny plane warts. 542 The regression is probably the result of cell-mediated immune mechanisms.543. and 544. The epidermodysplasia verruciformis induced by HPV-3 and HPV-10 is characterized by widely disseminated plane warts or large brownish plaques (see below). In these circumstances the warts persist as a result of impaired cell-mediated immunity. 464
There is hyperkeratosis and acanthosis, with vacuolation of the cells of the granular and upper malpighian layers. The stratum corneum has a ‘basket-weave’ appearance (Fig. 26.15). The dermis is usually normal. In spontaneously regressing warts there is a superficial lymphocytic infiltrate in the dermis with exocytosis of these cells into the epidermis. 540 This is accompanied by the death of single epidermal cells by the process of apoptosis. 545 Often more than one lymphocyte is in contact with a degenerating epidermal cell. These latter cells are shrunken, with eosinophilic cytoplasm and pyknotic nuclear remnants. Regressing plane warts lose their histological features.
Verruca plana. There are vacuolated cells in the upper epidermis and a basket-weave pattern in the overlying stratum corneum. (H & E)
Epidermodysplasia verruciformis (EV – OMIM 226400) is a rare autosomal recessive genodermatosis due to mutations in EVER1/TMC6 or EVER2/TMC8, two adjacent genes located at 17q25.546.547. and 548. These two genes encode transmembrane proteins located in the endoplasmic reticulum, which are likely to function as modifiers of ion transporters and to be involved in signal transduction. 548 The EVER genes are members of the transmembrane channel-like (TMC) gene family which comprises eight genes (TMC1 to 8). EVER1 and EVER2 are identical to the TMC6 and TMC8 genes, respectively. 548 There is a susceptibility to specific strains of HPV (the so-called β papillomaviruses), which lead to the development of disseminated plane wart-like lesions and, in some patients, lesions resembling pityriasis versicolor. Some of the HPV genotypes, mainly HPV-5, have oncogenic potential leading to the development of Bowen’s disease and squamous cell carcinoma, mainly on sun-exposed areas. Individuals with EV are not prone to other infections, either viral, bacterial, or fungal, or abnormally susceptible to other HPV infections, although concurrent HPV infection with non-EV strains has been reported. 549 Orth has called EV a primary deficiency of intrinsic immunity against certain papillomaviruses. 548
Only 75% of patients with EV carry mutations in the TMC6 or TMC8 genes. This suggests genetic heterogeneity with the involvement of other genes (see below).548. and 550. EV has also been reported in patients with HIV infection,551.552.553.554. and 555. and in the setting of GVHD, 556 and CD8+ T-cell lymphocytopenia. 557 These cases may be linked to the unmasking or growth of EV-HPV strains, or the possession of a haplotype that represents susceptibility alleles to EV-HPVs. Defective Fas function and variation of the perforin gene were found in another patient lacking TMC mutations. 558
EV usually begins in infancy or childhood. 559 More than 20 different human papillomavirus types (β papillomaviruses) have been incriminated, including HPV-3, 5, 8, 9, 10, 12, 14, 15, 17, 19–25, 28, 29, 36–38, 46, 47, 49, 50, and 59. 448 Two forms of the disease are currently recognized. 560 One form is induced by HPV-3, and sometimes HPV-10; these virus types are also responsible for plane warts. 464 Not surprisingly, this form is characterized by multiple plane warts, but the disease is distinguished by the persistence of the lesions, their wider distribution and the presence of plaques. The distinction between this type of EV and multiple plane warts is not always clear cut. Severe vegetating lesions are uncommon. 561 Some of the cases are familial. There may be disturbed cellular immune function.562.563.564. and 565. There is no tendency to malignant transformation in this form. Regression of the lesions has been reported. 560 The exact status of this type of EV is controversial.
The second form of EV is related to HPV-5, and sometimes HPV-8, 9, 14, 20, 24, 38, 47, and others.566.567.568. and 569. Two susceptibility loci have been mapped to chromosome regions 2p21–p24 and 17q25. 570 17q25 is the site of the EVER1/TMC6 and EVER2/TMC8 genes as mentioned above. This latter region also contains a susceptibility locus for psoriasis (PSORS2), which is of interest in view of the finding of some EV-related strains of HPV in patients with psoriasis (see below).571.572. and 573. The gene located at 2p21–p24 has not been identified, but it has been reported as a second locus in a French family with EV. 548 In addition, X-linked recessive inheritance (OMIM 305350) and vertical transmission have also been reported.574. and 575. In addition to the plane warts, reddish-brown patches and lesions resembling scaling pityriasis versicolor and seborrheic keratosis develop,576. and 577. with the eventual complication of Bowen’s disease578 or invasive squamous cell carcinoma. Malignant transformation, which occurs in 25–50% of patients with EV, depends on the oncogenic potential of the infecting virus.464. and 579. This is highest for HPV-5, followed by HPV-8.580. and 581. Carcinomas develop mainly in light-exposed lesions.582. and 583. Such tumors have a very low rate of metastasis, 566 although patients with advanced squamous cell carcinomas have a much higher rate. 584 Patients with this oncogenic form of EV have an altered natural killer-cell cytotoxic response. 566 p53 protein expression is common in lesional skin. 585 This form of EV has been reported in a patient who developed intestinal lymphoma. 586 Beta-papillomavirus DNA has been detected in some cutaneous squamous cell carcinomas in immunocompetent individuals. 587
Recently, an EV-like syndrome was reported in a patient with depressed cell-mediated immunity, primary lymphedema, disseminated warts, and anogenital dysplasia. 588 However, only non-EV-HPV strains were isolated from the warts and anogenital region. 588
Some of the HPV types involved in epidermodysplasia verruciformis have been implicated in other conditions. For example, HPV-8 has been found in actinic keratoses in patients without EV. 589 Other EV-related HPV strains have been reported in patients with non-melanoma skin cancer.590. and 591. HPV-5 has been found on the skin, and on hairs, of both normal and immunocompromised individuals and also in lesional skin of psoriasis.571.592.593.594.595. and 596. EV-HPV strains have been found in a large numbers of patients with HPV vulvitis, a disputed entity in which genital-mucosal HPV strains have not been detected. 597 These EV-HPV strains have also been detected in vulvar and vaginal melanomas. 598
Finally, neurological manifestations and isolated IgM deficiency have been reported in patients with epidermodysplasia verruciformis.599. and 600.
The use of sunscreens is recommended, particularly in those patients harboring oncogenic strains of HPV. Therapy with electrodesiccation, cryotherapy, topical retinoic acid, and surgery are generally unsatisfactory. 601 Treatment with a combination of retinoids and interferon alfa-2a has given only irregular and transient results.548. and 601. However, a recent trial of systemic low-dose isotretinoin maintained remission status in one patient with EV. 602 Treatments with imiquimod,603. and 604. tacalcitol ointment, 605 and oral cimetidine606 have also been reported. Remission of an EV-like eruption occurred in an HIV patient after the commencement of highly active antiretroviral therapy. 607 Radiotherapy has been used for large squamous cell carcinomas. 608
The cases induced by HPV-3 resemble plane warts. In the other form there is some variability in the changes seen. Some lesions may resemble plane warts, whereas others consist of thickened epidermis with swollen cells in the upper epidermis (Fig. 26.16 and Fig. 26.17). This latter change is a specific cytopathic effect seen in the various HPV types associated with epidermodysplasia verruciformis. Its full expression is characterized by large cells, sometimes in nests, in the granular and spinous layers. There is a conspicuous perinuclear halo. 609 The nucleoplasm is clear and the cytoplasm, which has a blue-gray pallor, contains keratohyaline granules of various sizes and shapes. 610 The horny layer is loose and has a basket-weave-like appearance. 610 Dysplastic epidermal cells may be seen. Changes of Bowen’s disease or squamous cell carcinoma may ultimately supervene.
Epidermodysplasia verruciformis. The epidermal changes resemble, somewhat, a condyloma acuminatum. Squamous cell carcinoma was present in a neighboring area. (H & E)
Epidermodysplasia verruciformis in a patient with HIV infection. Large pale keratinocytes are present. (H & E)
Although condyloma acuminatum is traditionally defined as a fleshy exophytic lesion of the anogenital region,612. and 613. it is now known that small inconspicuous lesions may occur on the penis,614.615. and 616. vulva, and cervix.617.618. and 619. The prevalence of HPV infection in the glans/corona region is significantly higher in uncircumcised men than in circumcised men. 620 Condylomas are usually sexually transmitted and spread rapidly. 463 Extensive lesions may occur in immunosuppressed persons. 621 Topical corticosteroids and tacrolimus used in the treatment of vulval dermatoses may lead to the reactivation of latent HPV after prolonged therapy.622. and 623. The incubation period is variable, but averages 2–3 months. 624 Lesions resembling condyloma acuminatum have been reported as an unusual complication of intertrigo, 625 and of healed herpes progenitalis. 626 A giant-sized condyloma, related to HPV-6 and 11, has been reported on the breast. 627 Children occasionally develop lesions, raising the issue of sexual abuse.628.629.630.631.632. and 633. However, it appears that HPV carriage is not uncommon in prepubertal girls, particularly if they have lichen sclerosus. 634 Condylomas in children regress spontaneously in more than 50% of cases. 635 This appears to result from a cell-mediated immune response, 636 involving CD8+ cytotoxic T-lymphocytes. 637 The virus attempts to evade immune recognition by down-regulating MHC-I surface expression. 637 Condylomas in all age groups are recurrent in up to one-third of cases. 638 This may be related to the persistence of HPV DNA in the dermis, on hairs, 596 or in underlying hair follicles.639. and 640. Although malignant transformation of condylomas of the anogenital region is rare, it is more common than in other types of warts, with the exception of epidermodysplasia verruciformis. 641 Giant condyloma acuminatum of Buschke–Lowenstein is now regarded as a variant of verrucous carcinoma,642.643.644. and 645. although this is still disputed by some. 646 Regression of a deeply infiltrating variant has been reported following long-term intralesional interferon alfa therapy. 647 Radical surgery has been practiced in some cases. 648
As only small amounts of virus are present, characterization of the etiological subtype of HPV involved in the causation of condylomas was made only in the last decade. HPV-6 and 11 are most commonly identified, but other types have been implicated, including HPV-2, 6, 11, 16, 18, 31, 33, 35, 39, 41–45, 51, 56, and 59.447.464.612.649.650. and 651. More than one HPV type may be present. 652 In one study, 83% of cases were positive for HPV-6 and/or 11, whereas 6% were positive for type 16. 447 HPV-16 and 18, and numerous other uncommon subtypes have oncogenic properties and are sometimes isolated from condylomas, although more usually HPV-16 is seen in association with bowenoid papulosis. 653 HPV oncogenesis is complex and involves genetic susceptibility, immune responses, and environmental and infectious cofactors. 654 The concurrence of condyloma acuminatum and bowenoid papulosis, and of condyloma acuminatum, HPV-31-positive Bowen’s disease, and coexisting extramammary Paget’s disease655 have been reported.655. and 656. Penile lesions, often subclinical, are frequently found in the sexual partners of women with cervical intraepithelial neoplasia (CIN), indicating that the male sexual partners of women with CIN might constitute a reservoir for high-risk HPV. 657
Most conventional therapies for condyloma acuminatum are traumatic and have high recurrence rates. 658 They include surgical electrodesiccation, cryotherapy, trichloroacetic acid, and podophyllotoxin and related substances. 633 A recent (2007) comparative study found that phototherapy following the topical application of 5-aminolevulinic acid is a more effective and safer treatment with a lower recurrence rate compared with CO2 laser therapy. 658 Refractory cases have been successfully treated with 5% imiquimod cream.659. and 660. Polyphenon E® ointment, a proprietary extract of green tea leaves, cleared about half of the external genital and perianal warts in a recent trial. 661 Green tea catechins exert antiviral activity.
There is marked acanthosis, with some papillomatosis and hyperkeratosis. Vacuolization of granular cells is not as prominent as in other varieties of warts, although there are usually some vacuolated koilocytes in the upper malpighian layer. Lesions resembling seborrheic keratoses may be seen; they usually contain human papillomavirus. 663 Small penile lesions may only show a slightly thickened granular layer. 664 Coarse keratohyaline granules may also be present. Langerhans cells are sometimes prominent. 665 Acantholysis has been reported in one case. 627
If the lesions are treated with podophyllum resin (podophyllin) 48 hours or so prior to removal, there are striking histological changes. These include pallor of the epidermis, numerous degenerate keratinocytes in the lower half of the epidermis, and a marked increase in this region in the number of mitotic figures. 666 Persistent lesions, resistant to treatment with the immune-response modifier imiquimod, have only rare factor XIIIa-positive dendrocytes in the upper dermis. 667 This feature may be responsible for low cytokine production and explain the resistance to treatment.
Papillomavirus common antigen can be detected in about 60% of lesions by immunoperoxidase techniques. 668 Its presence correlates with that of coarse keratohyaline granules and koilocytes. 668 More sophisticated techniques increase this detection to almost 100%. The presence of MIB-1 immunostaining in the nuclei of the upper two-thirds of the epidermis correlates with the presence of HPV in lesions in which the morphology is suggestive, but not diagnostic, of condyloma. 669
Langerhans cells in condyloma acuminatum show degenerative changes suggesting that they are functionally impaired. 670 They are also reduced in number.
Also known as Heck’s disease, focal epithelial hyperplasia (OMIM 229045) is characterized by multiple soft pink or white papules and confluent plaques, particularly on the mucosa of the lips and cheeks. 671 There is a high incidence among Inuits (Eskimos) and American Indians, but there are isolated reports of involvement in other races.672. and 673. Individuals with the HLA-DR allele HLA-DR4 (DRB1*0404) are at risk for the development of this disease. 674 Familial aggregation has been recorded. HPV-13 and 32 have been implicated in the etiology of this condition.465.674.675.676. and 677.
As for HPV infections at other sites, various treatments have been used including simple excision, electrocauterization, cryotherapy, and curettage. For more widespread lesions, acitretin, etretinate, interferon, methotrexate, and CO2-laser therapy have been used. 678
The involved mucosa is hyperplastic, with acanthosis and some clubbing of rete pegs. There is a characteristic pallor of epidermal cells, particularly in the upper layers. 679 There are often binucleate cells, but inclusion bodies are not found.
Bowenoid papulosis is the presence, usually on the genitalia, of solitary or multiple verruca-like papules or plaques having a close histological resemblance to Bowen’s disease.680. and 681. It has a predilection for sexually active young adults. If children develop lesions, the possibility of child sexual abuse should be considered. 682 In males it tends to involve the glans penis and also the foreskin, whereas in females the vulval lesions are often bilateral and pigmented. 683 This condition was first described as multicentric pigmented Bowen’s disease, 684 and several other terms were used before acceptance of the term ‘bowenoid papulosis’. 685 Although increasing in incidence, it is still a relatively uncommon condition. Cases localized to extragenital sites such as the neck,686. and 687. face, 688 and fingers689 have been reported.
Lesions are often resistant to treatment and may have a protracted course, particularly in those with depressed immunity. 690 Spontaneous regression is uncommon. Sometimes there is a history of a previous condyloma. In a small number of cases invasive carcinoma develops. This risk is greatest in women over the age of 40,683. and 691. but men are not immune.692. and 693. It has been suggested that a cocarcinogenic factor, as yet undetected, may be implicated in this malignant transformation. 694 Most cases of bowenoid papulosis are due to high-risk HPV-16, but in a small number HPV-18, 31, 33, 35, 39, and 53, or mixed infections, have been present.692.695.696.697.698. and 699. The HPV-16 strain has also been implicated in the pathogenesis of vulval carcinomas and cervical intraepithelial neoplasia.700. and 701. The latter condition is occasionally present in the sexual partner of patients with bowenoid papulosis of the penis. 683 The viral oncoproteins E6 and E7 contribute to oncogenesis in multiple ways. They also modulate the expression of p16 and human telomerase reverse transcriptase (hTERT). 702
Anogenital cancers have been associated, not only with the HPV types already mentioned, but also with some of the more recently identified types such as HPV-30, 31, 33, 45, 51, 52, 56, 58, 66, and 69. 448
Treatment of bowenoid papulosis usually involves locally destructive or ablative therapies such as surgical excision, shave excision, electrocoagulation, cryotherapy, and 5-fluorouracil. Imiquimod has also been used successfully.703. and 704. It remains to be seen by how much the new vaccine for HPV reduces the incidence of this disease.
The histological differentiation of bowenoid papulosis and Bowen’s disease is difficult, and may be impossible. Bowenoid papulosis is characterized by full-thickness epidermal atypia and loss of architecture. The basement membrane is intact. Mitoses are frequent, sometimes with abnormal forms. They are often in metaphase. Dyskeratotic cells are also present. True koilocytes are uncommon, 705 although partly vacuolated cells with a ‘koilocytotic aura’ are sometimes present (Fig. 26.18). The stratum corneum and granular cell layer often contain small inclusion-like bodies which are deeply basophilic, rounded, and sometimes surrounded by a halo. These bodies, together with the numerous metaphase mitoses, are the features that suggest a diagnosis of bowenoid papulosis rather than Bowen’s disease itself.
Bowenoid papulosis. There are atypical keratinocytes throughout the full thickness of the epidermis with several mitoses in metaphase. Small basophilic inclusions are present in the granular layer. (H & E)
The dermis usually contains a mild, superficial infiltrate of lymphocytes, often with perivascular accentuation. Patchy interface changes are sometimes present. Amyloid has also been reported in the papillary dermis. 706
Bowenoid papulosis is commonly classified by gynecological pathologists as vulvar intraepithelial neoplasia (VIN) III. 691
The Parvoviridae are single-stranded DNA viruses and among the smallest known DNA-containing viruses to infect mammalian cells. Parvovirus B19, which belongs to the genus Erythrovirus, is the only known human pathogen in this family.
Parvovirus infection occurs mainly in school-aged children and teenagers. Approximately 80% of the community is immune to the virus by the age of 50 years. 707 It can produce an influenza-like illness, miscarriages, fetal hydrops, neonatal angioedema, 708 polyarthritis, aplastic crises, pure red cell aplasia, 709 purpura, a generalized petechial eruption, 710 a petechial rash of the lower extremities, 711 a ‘bathing trunk’ exanthem,712.713. and 714. vasculitis, 715 erythema multiforme, 716 follicular purpuric papules with a baboon-syndrome-like distribution, 717 dermatomyositis, 718 a Sweet’s syndrome-like eruption, 718 lupus erythematosus-like syndromes,716. and 718. an asymptomatic papular eruption, livedo reticularis, 719 cutaneous necrosis, 720 acral pruritus, 721 the papular-purpuric (petechial) ‘gloves-and-socks’ syndrome, 722 and erythema infectiosum (fifth disease).723.724.725.726. and 727. A Degos-like presentation has been reported; it resulted from an underlying endothelialitis. 728 There is an increased prevalence of viral DNA in systemic sclerosis skin. 729
The papular-purpuric ‘gloves-and-socks’ syndrome is a self-limited infection characterized by pruritic, erythematous papules with petechiae and edema involving predominantly the hands and feet. 730 Fever, arthralgias, and oral lesions may be present. Lymphangitis is a rare manifestation. 731 This exanthem usually occurs in adults, but childhood cases have been recorded.732. and 733. It may represent a non-specific manifestation of several viral infections. 734 Although parvovirus B19 has been implicated most often, there are reports of a similar illness following measles virus, Epstein–Barr virus, human herpesvirus-6, human herpesvirus-7, hepatitis B infection, 735 coxsackievirus B6, and cytomegalovirus.736. and 737. The simultaneous occurrence of parvovirus B19 and human herpesvirus-7 has been reported in a familial setting. 733 A mother and daughter have also been involved. 738 In immunocompromised patients, persistent skin lesions and anemia often develop. 739 Serological confirmation can be used to confirm the diagnosis. 740 Approximately 50% of the adult population is immune. 741 It should not be confused with the purple glove syndrome resulting from the intravenous injection of phenytoin into a small vein on the dorsal hand. 742
Erythema infectiosum (fifth disease or ‘slapped-cheek’ disease) is an exanthem which may be difficult to distinguish from rubella. 743 A distinctive well-marginated rash often appears on the cheeks a few days after the onset of prodromal symptoms. The rash usually becomes more generalized after a few days.
Parvovirus B19 is spread by a respiratory droplet and has an incubation period of 5–14 days. 741 The receptor for the virus is the erythrocyte P antigen which is also expressed on endothelial cells. The virus has been demonstrated in endothelial cells in lesional skin by several methods, including PCR.744. and 745. It has also been identified in keratinocytes. 746 Parvovirus B19 has been reported as producing four distinct clinical presentations in the one family. 741 This suggests that the immunological response to the virus may play a role in determining the clinical presentation. 747
Because infection in healthy children and adults is self-limited, no specific therapy is warranted. 707 It should be remembered that parenteral transmission of the disease can occur via the transfusion of blood products from blood donated during the viremic stage of the illness. 707
The changes resemble other viral infections with a mild, usually tight perivascular infiltrate of lymphocytes, mild exocytosis of lymphocytes, and mild basal vacuolar change with occasional apoptotic keratinocytes, giving a so-called interface dermatitis. 748 Extravasation of red cells is often present. At times, the appearances are those of a lichenoid lymphocytic vasculitis (see p. 608) but fibrin is rarely present. 749 Eosinophils and occasional neutrophils may be present. Interstitial lymphocytes and histiocytes have been described, resembling incomplete granuloma annulare, 718 but this may be a late manifestation of a previous lymphocytic vasculitis in which a hypercellular interstitium (‘busy’ dermis) and mucin may be present. Perineuritis was also present in a patient with the papular-purpuric gloves-and-socks syndrome associated with mononeuritis multiplex. 749
The Picornaviridae are an important family of RNA viruses which includes the poliovirus, the coxsackievirus, and ECHOvirus. The latter two viruses are now the most common cause of exanthems in children. 1 Most of the cutaneous manifestations are transient macular or maculopapular lesions in the course of an obvious viral illness, and biopsies are rarely taken. As a result, very little is known of the histology. Urticarial and vesicular lesions have also been reported. 750 The most important of the vesicular group is so-called ‘hand, foot and mouth disease’, caused mostly by coxsackievirus A16. This virus has also been associated with an eruption resembling that of the Gianotti–Crosti syndrome, 751 although this condition is more usually associated with hepatitis B infection (see below). 752
Most cases are caused by coxsackievirus A16, although other types have also been implicated. Cases associated with epidemics of enterovirus 71 may develop serious systemic complications. 753 The disease is a febrile illness characterized by vesicles in the anterior parts of the mouth and on the hands and feet. 754 A case without oral lesions has been reported. 753 The vesicles are usually small and they may be sparse.
Acyclovir has been used to treat patients with this condition but as enteroviruses lack thymidine kinase, this agent is not suitable for cases with this etiology. Pleconaril, which has specific action on the viral capsid of enteroviruses, may prove to be effective. 753
There are intraepidermal vesicles with prominent reticular degeneration, and sometimes a few ballooned cells in the base. There are no multinucleate cells or inclusion bodies. Similar changes, usually without ballooning, are seen in the vesicular lesions of other viruses in this family. There is sometimes the additional feature of papillary dermal edema and a mild perivascular inflammatory infiltrate.
The family Togaviridae includes the alphaviruses, formerly known as group A arboviruses. This group includes three mosquito-borne equine encephalitis viruses as well as several so-called ‘Old World’ species such as Chikungunya, O’nyong-nyong, Sindbis, Ross River, and Barmah Forest. Rubella (German measles) also belongs to this family, but because it is serologically distinct from the alphaviruses and requires no vector for its transmission it has been placed in the genus Rubivirus.
Rubella produces an exanthematous eruption but it is best known for its causation of the congenital rubella syndrome that results from infection early in pregnancy. Since the introduction of a live-attenuated rubella vaccine in 1969, no large rubella epidemics have occurred in countries where the vaccine is widely used and the congenital rubella syndrome has virtually disappeared.
The alphaviruses tend to present with fever, arthralgia, some myalgia, and skin rashes but the latter may be very mild and transient. Some variability in presentation occurs as illustrated by the recent report from south India that reviewed the cutaneous manifestations of 145 patients with chikungunya fever, 755 a disease which has recently increased dramatically in incidence and geographic extent. 756 A maculopapular eruption was present in a third of patients. Other findings were pigmentation, which often appeared de novo, intertriginous aphthous-like ulcers and, in a small number of cases, a vesiculobullous eruption. 755 Cutaneous ulcers may also occur in this condition. 757 A tight superficial perivascular infiltrate of lymphocytes is usually present; a focal mild lichenoid reaction is occasionally seen as well. 755
Experimentally, Langerhans cells migrate from the skin to local lymph nodes following cutaneous infection. 758 This migration appears to be important in the development of an immune reaction to the virus. Large, atypical lymphoid cells have been reported in a perifollicular lymphohistiocytic infiltrate in a papular eruption in a patient with Sindbis infection. 759
Brief mention will be made of Ross River and Barmah Forest viruses. Although limited in their extent to Australia and Oceana, the author has had some experience with them. They serve as a prototype for the other Togaviridae.
The Ross River and Barmah Forest viruses are alphaviruses of the family Togaviridae. The Ross River virus was isolated by Doherty and colleagues from the mosquito Aedes vigilax trapped along the Ross River in northern Queensland, while the Barmah Forest virus was also isolated from the mosquitoes in the Barmah Forest in northern Victoria, Australia, in 1974. 760 While found mostly in Australia, the Ross River virus has caused major epidemics in Fiji and Samoa. 760 Ross River viral infections are nearly three times as common as those due to Barmah Forest virus, but the latter is increasing in incidence. 761 Climate change is predicted to cause an increase, not only in these viral infections, but also in infections by some of the other alphaviruses.
After an incubation period of 7–9 days, the presenting symptoms are joint pains, fever, and a rash.762. and 763. Sometimes the polyarthritis is debilitating. Myalgia and fever may persist for 6 months. 760 The skin rash is a diffuse maculopapular erythematous eruption which is seen predominantly on the limbs and trunk. It lasts for several days. A rash is more common with Barmah Forest virus.760. and 764.
As with most viral infections, few reports have described the cutaneous findings in these two diseases. Some cases have exhibited a mild, but tight, superficial perivascular infiltrate of lymphocytes, and macrophages; eosinophils may also be present in Barmah Forest viral infections (personal observations). There may be mild perivascular edema and some red cell extravasation in purpuric lesions. 763 A lichenoid lymphocytic vasculitis is another manifestation of these diseases, but whether it is correlated with persistence of the virus is currently unknown (Fig. 26.19).
Barmah Forest virus infection. (A) There is a lichenoid lymphocytic vasculitis with an unusual amount of subepidermal edema. (B) Rare eosinophils are in the tight perivascular infiltrate. (H & E)
The family Flaviviridae contains three antigenically distinct genera: Flavivirus, Pestivirus, and Hepacivirus. The genus Flavivirus contains 30 human pathogens including the viruses that cause yellow fever, West Nile fever/encephalitis, 765 St Louis encephalitis, Japanese encephalitis, and dengue hemorrhagic fever. The Pestivirus genus is not of human importance, while the genus Hepacivirus contains the organism that causes hepatitis C. The public health burden of several flaviviral infections has been such that attention was given many years ago to the development of vaccines to control the diseases. Where available, they have led to a significant decrease in the incidence of that disease. There are four serotypes of the dengue virus making the development of a vaccine difficult. Furthermore, immunity against one serotype can lead to more serious disease should infection subsequently occur with another serotype.
The following diseases will be considered in the account that follows:
• West Nile fever/encephalitis
• dengue fever
• hepatitis C.
West Nile virus is endemic in East Africa, but it first appeared in North America (New York City) in 1999. It has spread steadily across the country since that time. 5 There were 244 deaths in the United States for the year 2003. 766 The main reservoir for the virus is birds of the crow family. It is transmitted by culicine mosquitoes. 767 Cases are now being seen in Europe, Asia and, very rarely, in Australia (from the Kunjin subtype).
Approximately 80% of persons infected with West Nile virus have no signs or symptoms. 5 Only a small number, estimated to be 1 in 150, develop severe neurological disease, and this occurs mainly in individuals over 50 years of age. Multifocal chorioretinitis appears to be a specific marker for West Nile virus infection, particularly in patients presenting with a meningoencephalitis. 768 After an incubation period of 3–14 days, those individuals with overt disease will develop a febrile illness of sudden onset, often accompanied by malaise, eye pain, headache, and myalgia.
The rash consists of non-blanching, punctuate, erythematous macules and papules. It occurs predominantly on the extremities, particularly the palms and soles, and spreads centripetally. Some tick-borne diseases can mimic this pattern.766. and 769.
Currently there is no specific drug treatment or vaccine against this infection. 767
Few reports have appeared on the histopathological findings of the skin rash. In one case, there was a sparse superficial perivascular infiltrate of lymphocytes, a feature commonly seen in viral exanthems. 769
Up to one hundred million cases of dengue fever occur annually worldwide, making it one of the most important viral diseases in the world. 5 The more severe hemorrhagic form affects about 250 000 individuals each year. 770 Dengue fever is endemic in tropical and subtropical regions of Asia and Africa, particularly in southeast Asia, India, and Western Pacific countries. 771 The disease is spreading to other countries with 609 000 cases in the Americas in the year 2001. 5 It is now a common cause of an acute febrile illness in travelers returning from endemic areas. 772 The mosquito, particularly Aedes aegypti, which breeds in any urban stagnant water, is both the reservoir and vector of the disease.
There at least four serotypes of dengue virus and once infected with one serotype lifelong immunity to that serotype develops. Immunity to other serotypes is limited and transient, and if there is subsequent infection with a different serotype, the patient is at risk of developing a more severe clinical subtype of the disease, either dengue hemorrhagic fever (DHF) or dengue shock syndrome (DSS). Viral replication appears to occur in dendritic cells, monocytes, and possibly circulating lymphoid cells. 773 Reinfection, even by a different serotype, results in the activation of memory-T cells with the production of a cascade of inflammatory cytokines. 773 The exact mechanism by which reinfection with a different serotype of the virus results in damage to liver and lung cells, and vascular endothelium is controversial. Antibody-dependent enhancement is one postulated mechanism. 773
Three clinical subtypes of the disease occur – dengue fever (DF), DHF, and DSS. Asymptomatic infection also occurs. 774 DF is the least severe and the most common. 775 DHF has hemorrhagic manifestations including hematuria and melena. 774 Up to 30% of cases of DHF may progress to the more severe DSS as a consequence of increased vascular permeability leading to hypovolemia.
Clinical features of DF consist of the sudden onset of high fevers, myalgia, retro-orbital pain, severe headaches, and rash. Initially there is flushing erythema of the face, neck, and chest. This is followed a few days later by a more diffuse, erythematous, maculopapular eruption. 772 It is characterized by spared areas of normal skin within the diffuse erythema. 775
The diagnosis of DF and its clinical variants can be made serologically, by viral isolation, or by the detection of viral antigens by PCR-based methods.
The prevention of DF rests with protective clothing, mosquito nets, and insect repellents. There is concern that patients vaccinated against one serotype and subsequently infected by another are at risk of developing the severe forms of the disease. 775 Accordingly, a tetravalent vaccine is required, but research is continuing on its development. 775
There is usually a mild perivascular infiltrate of lymphocytes in the superficial dermis; some exocytosis of these cells may be present. 775 There is variable red cell extravasation, particularly in the hemorrhagic form of the disease. The histopathological changes are of no prognostic value in predicting the course of the disease. 776
Hepatitis C virus is an RNA virus, approximately 50 nm in diameter. It belongs to the genus Hepacivirus of the family Flaviviridae. Since its discovery in 1989, the hepatitis C virus has assumed great clinical importance as a cause of chronic hepatitis. Other organs may be involved, usually through immunological mechanisms.777.778.779. and 780. Skin disorders have been reported in up to 15% of patients afflicted with this virus. Most of these conditions are discussed in more detail elsewhere. They include:
• vasculitis (mainly cryoglobulin-associated vasculitis or polyarteritis nodosa) 781
• erythema nodosum (see p. 460)
• erythema multiforme (see p. 51)
• Behçet’s syndrome (see p. 224)
• necrolytic acral erythema (see p. 490)
• red fingers syndrome788
• symmetric polyarthritis with livedo reticularis789
This family of RNA viruses includes the following genera: Rubulavirus (mumps virus), Respirovirus (human parainfluenza virus types 1 and 3), Morbillivirus (measles virus), Pneumovirus (human respiratory syncytial virus), and several others.
Measles, an acute infection caused by the rubeola virus of the genus Morbillivirus, is highly contagious and usually seen in children. The pathogenesis of measles is said to begin with infection of the respiratory epithelium. The virus then spreads to lymph nodes, followed by viremia and infection of other organs including the skin. 793 Koplik spots are a characteristic feature of measles, and they precede the onset of the more widespread rash. Uncommonly a follicular rash may occur. 793 A biopsy from the rash of measles shows epidermal spongiosis and mild vesiculation, with scattered shrunken and degenerate keratinocytes.794. and 795. This latter feature may be prominent in patients with AIDS. 795 Occasional multinucleate epithelial giant cells may be seen in the upper epidermis and in hair follicles and acrosyringial cells.793. and 796.
Human T-cell lymphotrophic virus types 1 and 2 (HTLV-1 and HTLV-2) are members of the Delta genus of the family Retroviridae. HTLV-1 is the more important of the two species, as the majority of patients with HTLV-2 infection remain asymptomatic.
Both genera originated from primates; they are closely related to simian immunodeficiency virus and simian T-cell lymphotrophic virus, respectively.
The human immunodeficiency virus (HIV) is a retrovirus that infects and destroys CD4+ T lymphocytes by apoptosis, 797 with resulting disturbances in cellular immune function.798. and 799. It also infects cells of the central nervous system, producing dementia and motor disturbances. Lymphadenopathy is another manifestation of HIV infection. The acquired immunodeficiency syndrome (AIDS) is one manifestation of a variety of disorders caused by infection with this virus. AIDS is found most commonly in male homosexuals, in hemophiliacs who have received infected blood, in intravenous drug users, and in parts of Africa, where heterosexual transmission is important. For example, more than 10% of the population in Cameroon, Africa are currently living with HIV/AIDS. 800 According to the 2006 UNAIDS survey, an estimated 38.6 million people worldwide were living with HIV infection or AIDS at the end of 2005. 801 Globally, and in every region, the proportion of women with HIV infection has increased dramatically. 802 In sub-Saharan Africa, close to 60% of all cases involve women, while in the United States, the proportion of women amongst newly infected or AIDS diagnoses increased from 15% before 1995 to 26% in 2006. 802 Genital ulcer disease in women is a potent facilitator of HIV transmission. 803 Viremia is lifelong.
Kaposi’s sarcoma was the first cutaneous manifestation of AIDS to be reported (see p. 913). It is found in up to one-third of patients, and is an adverse prognostic factor. Its incidence is lower in some non-Western countries, such as India. 804 The role of human herpesvirus-8 in its etiology is discussed elsewhere (see p. 619). Many other cutaneous effects have been described in recent years. These fall into three broad categories: infections, usually of an opportunistic nature, neoplasms,805. and 806. and non-infectious dermatoses.807.808.809.810.811.812.813.814.815.816.817. and 818. Malignant melanoma and squamous cell carcinoma are examples of cutaneous malignancies that have a more aggressive course in patients with HIV infection. 819 Lymphoma is increased in incidence.820. and 821. These diseases are detailed in Table 26.1. Detailed references can be found in a number of reviews of this subject.819.822.823.824.825.826.827. and 828.
Opportunistic infections, and some cancers, 829 have decreased in patients with HIV since the introduction in 1997 of so-called ‘highly active antiretroviral therapy’ (HAART), now called simply antiretroviral therapy (ART). This therapy typically consists of 3 or more medications from the current armamentarium of 20 anti-HIV drugs in five separate classes. 819 Viral titers fall with this therapy and there is a progressive increase in CD4+ cells. 830 Over time, reappearance of the complete T-cell repertoire occurs. 831
Other skin conditions that seem to improve or decline in incidence with HAART include Kaposi’s sarcoma, 819 eosinophilic folliculitis, candidiasis, dermatophyte infections, herpes simplex infections, Norwegian scabies, and verrucous lesions of herpes zoster. 831 The initiation of HAART may exacerbate herpes zoster, 830 mycobacterial infections, 832 and other granulomatous diseases. 833 It also increases the odds of developing photosensitivity, and increases the incidence of molluscum contagiosum. 830 Such diseases are part of the spectrum of the so-called immune reconstitution syndrome. 833 Complications of HAART, using protease inhibitors, include a lipodystrophy (see p. 471) and paronychia. 834
Early signs of HIV infection may be a roseola-like rash associated with recent infection and seroconversion,835.836.837. and 838. or the development of psoriasis/seborrheic dermatitis, 839 pruritic lesions,840. and 841. herpes simplex and zoster, a chronic acneiform folliculitis, oral candidosis, tinea, or impetigo of the neck and beard region.842.843. and 844. Seborrhoeic dermatitis, photosensitivity, and xerosis are three of the more persistent cutaneous manifestations.845. and 846. The incidence and severity of skin disease is a good indicator of the underlying immune status of the patient.847. and 848. Pregnancy does not seem to increase the prevalence of cutaneous diseases in patients with HIV infection. 849 Infections in immunocompromised patients may differ in severity and other clinical features from those in normal hosts.850.851. and 852.
A unique manifestation of HIV infection is oral ‘hairy’ leukoplakia, which is characterized by raised, poorly demarcated projections on the lateral borders of the tongue, resulting in a corrugated or ‘hairy’ surface.853.854. and 855. It has been observed in nearly 25% of patients with HIV-associated skin disorders.826. and 856. It is induced by Epstein–Barr virus but human papillomavirus has also been identified in some biopsy specimens. 857Candida is frequently present. The development of oral ‘hairy’ leukoplakia in patients with HIV infection indicates advanced immunosuppression. 856 In other clinical settings of immunosuppression, however, oral ‘hairy’ leukoplakia is quite rare.
Pruritic papular eruption is the most common cutaneous manifestation in HIV-infected patients in most series.858.859. and 860. It consists of chronic, pruritic, discrete papules on the trunk, extremities, and face. 861 Excoriation is often present. The incidence is increased in patients with low CD4 cell counts. 860 This eruption is distinct from the rare widespread, pruritic disorder, often with pigment changes, seen in late stage HIV infection and characterized by atypical lymphocytes in the skin, which are CD8+. This pseudo-Sézary syndrome rarely progresses to frank lymphoma. 862 Pruritic papular eruption also needs to be distinguished from follicular eruptions, which are also increased in HIV-infected individuals. 861
Changes in the immune system, including T-cell function, antigen response, and shifting cytokine expression, as well as a propensity for autoimmune reactions, appear to underlie the skin changes occurring in AIDS.863. and 864. Their occurrence correlates with low CD4 lymphocyte counts.865.866.867. and 868. Cutaneous infections and a pruritic eruption have been reported in association with a ‘syndrome’ characterized by idiopathic CD4+ lymphocytopenia but no evidence of HIV infection.869.870. and 871.
Various inflammatory dermatoses occur in patients infected with the human immunodeficiency virus type 1 (HIV-1). Subtle differences have been found in the expression of the diseases in these patients compared to patients without HIV infection. The number of neutrophils and eosinophils in inflammatory infiltrates may increase during the course of the disease. Plasma cells are sometimes present in diseases in which they are not usually found. Spotty parakeratosis is also more common. Apoptotic keratinocytes, often with an adjacent lymphocyte, are sometimes seen. At times the appearances resemble those seen in graft-versus-host disease (see p. 55). T cells, negative for CD7, are increased in cutaneous infiltrates. 872 This class of lymphocyte is epidermotropic. Increased numbers of CD30+ cells appear in the infiltrates of inflammatory dermatoses in later stages of AIDS. 873
The pruritic papular eruption of HIV infection shows variable features. There is a superficial perivascular infiltrate of lymphocytes, often with some eosinophils. Neutrophils and plasma cells are less common.874. and 875. Extension of the infiltrate around the deep plexus and appendages also occurs. Factor XIIIa-positive cells are usually increased. Dermal fibrosis and changes suggestive of early ‘necrobiosis’ are often present.
In the acute exanthem of HIV infection (seroconversion) there is a tight perivascular infiltrate of lymphocytes. Epidermal changes are usually mild, but may include spongiosis, vacuolar alteration, scattered apoptotic keratinocytes, or epidermal necrosis.876.877.878. and 879. Folliculitis is uncommon. In many cases the changes resemble those seen in other viral exanthems.
In oral ‘hairy’ leukoplakia there is some acanthosis and parakeratosis. Large pale-staining cells resembling koilocytes are present in the upper stratum malpighii. Candida is frequently present in the keratin projections on the surface.
The human retrovirus HTLV-1 was first isolated in 1980. It is found in parts of Japan, the Caribbean, and sub-Saharan Africa. Immigration has led to its dissemination to other countries, although its seroprevalence in the United States is very low. 880 This virus was originally found to be the cause of adult T-cell leukemia/lymphoma (see p. 981), but recently it has been associated with infective dermatitis of children, a condition with close clinical and histopathological similarities to atopic dermatitis. 881 Both diseases are a chronic dermatitis with a propensity for colonization with Staphylococcus aureus and β-hemolytic streptococci. 882 Infective dermatitis presents as an eczema of the scalp, axillae and groins, external ear and retroauricular region. Dermatopathic lymphadenopathy is common.880.882. and 883. Erythema multiforme-like, and ichthyosis-like lesions have also been reported.884. and 885. Breastfeeding appears to be the origin of the infection in children. 881
Co-infection with HIV is sometimes found. These patients have a higher risk of inflammatory dermatoses than do patients with either disease alone. 887
The diseases that follow are caused by RNA viruses, or presumptive viruses with the exception of hepatitis B, which is caused by a DNA virus of the family Hepadnaviridae. It is considered here so that its description is included with hepatitis A.
Some of the viruses still to be considered have a limited regional localization and their cutaneous manifestations are of little clinical importance in comparison to the encephalitis that accompanies some of these infections. Several families of RNA viruses will be considered briefly in this introduction. They include.
• Arenaviridae (including Lassa virus)
• Filoviridae (including Ebola virus)
• Bunyaviridae (including hantaviruses)
• Reoviridae (including rotavirus).
The Arenaviridae are RNA viruses that cause asymptomatic or mild disease or a more severe clinical illness. 5 The latter category includes lymphocytic choriomeningitis, the first recognized cause of aseptic meningitis in humans. Other arenaviruses are Lassa virus, Junin virus, Tamiami virus, Machupo virus, and Whitewater Arroyo virus. They cause a hemorrhagic fever with a mortality of up 15%. 5 Contact with rodent excreta causes human disease. Vaccines for some of these viruses are under development. 5
The Filoviridae are contained in a single genus, Filovirus, but it is separated into two genotypes, Marburg and Ebola. Marburg and Ebola viruses both cause severe hemorrhagic fevers. 5 They are indigenous to Africa. Ebola virus spreads to close contacts; it is particularly likely to spread to healthcare workers. The cutaneous manifestations include a maculopapular centripetal rash associated with varying degrees of erythema, which desquamates after 5–7 days. 5 No virus-specific treatment exists.
The Bunyaviridae family encompasses about 300 different viruses, two of which are associated with hemorrhagic fevers and mucocutaneous lesions: Rift Valley fever and Crimean-Congo hemorrhagic fever. Sporadic outbreaks occur in humans. Included in this family is the genus Hantavirus, which includes viruses specific to rodent genera worldwide. The virus is transmitted by aerosols of rodent excreta, saliva, and urine. They are listed in the excellent review of this subject by Lupi and Tyring. 5
The family Reoviridae includes the genus Rotavirus, the most common cause of severe gastroenteritis in children below the age of 5 years. The primary mode of transmission is fecal–oral. It has been associated, rarely, with exanthema, Gianotti–Crosti syndrome, and acute infantile hemorrhagic edema. 888 This family also contains the orbiviruses, which include the agent of Colorado tick fever in humans.
The remainder of this section will include a discussion of the following diseases:
• Hepatitis A
• Hepatitis B (including Gianotti–Crosti syndrome)
• Kikuchi’s disease
• Asymmetric periflexural exanthema.
Skin manifestations associated with infection by hepatitis A virus have been rarely reported. They include a photo-accentuated eruption, accompanied by the deposition of IgA in endothelial cells of the upper dermis. 889 The Gianotti–Crosti syndrome (see below) and a vasculitis are other rare manifestations.
The dermatological manifestations of the hepatitis B virus include:
Gianotti–Crosti syndrome is characterized by a non-relapsing, symmetrical, erythematopapular rash, lasting about 3 weeks and localized to the face and limbs, with the addition sometimes of lymphadenopathy and acute hepatitis, usually anicteric.897.898.899. and 900. Truncal lesions sometimes occur. 901 It primarily affects children between 2 and 6 years of age. 900 The syndrome has also been associated with many other viruses, including hepatitis A, 902 cytomegalovirus, 903 herpesvirus-6, 413 Epstein–Barr virus,904. and 905. adenovirus, 903 rotavirus,888. and 906. parainfluenza virus, 907 and coxsackievirus.908. and 909. It has also been reported following various immunizations910.911.912.913. and 914. and following Mycoplasma pneumoniae infection. 915 The histology can show a characteristic mixture of three tissue reactions: spongiotic, lichenoid, and lymphocytic vasculitis. Usually the lichenoid features consist of mild basal vacuolar change; rarely, this is a dominant feature. 916 Red cell extravasation and papillary dermal edema are other features.
Kikuchi’s disease is a rare self-limiting lymphoproliferative disorder characterized by acute or subacute necrotizing lymphadenitis. 918 It affects predominantly young adult females. 919 It is of presumptive viral origin, based on its clinical features and course. Most cases have been reported from Japan, but cases from other countries continue to be reported.920. and 921. Viruses implicated have included HHV-6, HHV-8, 922 HTLV-1, 923 EBV,401. and 402. and cytomegalovirus. However, in a study of archival tissue of 34 cases, HHV-8 and EBV were not detected in any case. 924 In a more recent study the HHV-8 genome but not protein was detected in a small proportion of cases. 925 Cutaneous involvement occurs in about 30% of patients. The eruption may be morbilliform, urticarial, maculopular, or disseminated erythema.926. and 927. Cervical lymphadenopathy and fever are important clinical features. 928 Kikuchi’s disease has been reported in association with cutaneous lupus erythematosus929.930.931. and 932. and Still’s disease. 933 A t(2:16) chromosomal translocation was present in one case. 918 Immediate remission of the disease has followed the use of oral ciprofloxacin. 934
The histological changes may show some resemblance to lupus erythematosus, with a lichenoid (interface) reaction with basal vacuolar change and some apoptotic keratinocytes. There is a variable superficial and deep perivascular infiltrate of lymphocytes and histiocytes in the dermis. Extension into the subcutis is not uncommon.
Subepidermal edema is often present and histiocytes containing karyorrhectic nuclear debris are often seen in the base of the edema. 936 A CD68 stain confirms that many of the cells that initially seem to be lymphocytes are of histiocytic lineage. 936 Cells with a cleaved or deformed nucleus, sometimes resembling Reed–Sternberg cells, have been reported. There is a conspicuous absence of neutrophils and a paucity of plasma cells. 936 A similar clinical picture can be seen in some adverse antibiotic-induced eruptions associated with Epstein–Barr virus infection. 937
The lymph nodes show paracortical areas of necrosis with abundant karyorrhectic debris. 928 Numerous histiocytes are present in these areas as well as large lymphoid cells.
Also known as unilateral laterothoracic exanthem, asymmetric periflexural exanthem is of presumptive viral etiology, although no virus has yet been consistently detected by various methods.938. and 939. Parvovirus B19 has been detected serologically in several cases.940. and 941. There are sometimes upper respiratory tract prodromes. 940 It begins as a unilateral eruption close to the axilla with centrifugal spread. 942 It is typically described as a maculopapular scarlatiniform eruption or an eczematous dermatitis. 940 It may be mildly pruritic. Regional lymphadenopathy is common. 943 There is spontaneous resolution after several weeks. This disorder is rare after childhood;944.945. and 946. the youngest patient to be reported is a 4-year-old female infant. 943
There is a superficial perivascular infiltrate of lymphocytes which often forms a tight cuff around the vessels. Some authors have described a lymphocytic infiltrate around eccrine ducts with mild miliarial spongiosis and exocytosis of lymphocytes into the acrosyringium.942. and 947. Mild lichenoid changes may be present in late lesions.