Vascular Tumors: Introduction
Vascular anomalies are common birthmarks.1 Their classification has often been problematic, with contradictory and confusing descriptive nomenclature. A classification system first proposed by Mulliken and Glowacki was revised in 1996 by the International Society for the Study of Vascular Anomalies based on clinical, radiologic, and hemodynamic characteristics, into vascular malformations and vascular tumors.2 Vascular malformations (see Chapter 172) are errors of morphogenesis whereas hemangiomas and other vascular tumors grow by cellular proliferation.
There are several types of vascular tumors, many of which occur in childhood. These tumors have also had confusing nosology,3 with descriptive but imprecise terminology such as strawberry, capillary, and cavernous. Several types of hemangiomas have been described (infantile, rapidly involuting congenital, lobular capillary, etc.), so whenever possible, hemangioma should not be used as a stand-alone noun but rather qualified by an adjective based on the specific condition.
Infantile Hemangiomas
|
Infantile hemangiomas (IH) are the most common benign tumors of childhood, occurring in approximately 4% of children by 1 year of age.4,5 In contrast to other types of hemangiomas and vascular malformations, IH have a characteristic proliferative phase followed by a slower involution phase. They are more common in females (2–5:1 ratio) and in premature infants, especially those weighing less than 2,500 g.6,7 Other risk factors are Caucasian race, multiple gestation pregnancy, and maternal age greater than 30 years. Preterm infants are more likely to have multiple tumors, and the sex ratio is less skewed toward females.8 Contrary to prior observations, newer prospective studies do not support chorionic villus sampling as a risk factor.7,8
IH are primarily composed of endothelial cells but also contain fibroblasts, pericytes, interstitial cells, and mast cells. Although the precise pathophysiologic mechanisms of the growth and involution of endothelial cells remains unknown, several recent discoveries have advanced our understanding of IH.9–13 The patterns found in segmental hemangiomas suggest at least some hemangiomas occur via developmental error as early as 4–6 weeks of gestation.14 IH only occur in humans, and adequate animal or laboratory models have been difficult to develop.
The recognition that a glucose transporter protein, GLUT1, is expressed in all stages of hemangioma maturation spurred new hypotheses on the pathogenesis of IH.15,16 GLUT1 expression in absent in the normal cutaneous vasculature but is found in placental blood vessels as well as in other so-called barrier tissues such as the blood–brain barrier. This, together with other immunohistochemical markers shared by IH and human placenta (FcγRII, merosin, and LeY), and the similar gene expression profiles found on DNA-based microarrays led to speculation that these tumors are of placental origin from either embolized placental cells or invading angioblasts that have differentiated toward a placental phenotype.16,17 IH lack a villous architecture and do not express known placental trophoblastic markers suggesting that they are not placental emboli.18 In addition, a recent study determined that hemangioma endothelial cells are of fetal, not maternal origin.19 Further investigations into the cellular origin of hemangioma endothelial cells have demonstrated that these cells have features of immature mesenchymal cells. They have features similar to an early embryologic vessel, the cardinal vein20 and express CD133, a primitive cell marker, during proliferation.21,22 Implantation into immunodeficient mice of CD133+ cells isolated from IH gives rise to GLUT1+ vessels that later diminish and are replaced by adipocytes.23 While not a perfect replica of IH growth, this model merits attention and additional study.
Additionally, while previous work suggested that IH arise because of aberrations in angiogenesis, recent investigations show that IH are not simply cutaneous capillaries with excessive growth, but more likely represent de novo vasculogenesis in the skin and other sites. Alterations in pathways that negatively control vascular endothelial growth factor receptor 2 (VEGFR2) signaling in vascular endothelial cells appear to play an important role in IH development and their rapid growth. In some patients germline mutations in VEGFR2 or tumor endothelial marker 8 (TEM8) lead to these signaling abnormalities.24,25 In vitro studies indicate that hypoxia and estrogen synergistically enhance hemangioma proliferation.21 Some studies have demonstrated evidence of clonality in hemangiomas, but further research is needed to demonstrate whether these tumors, particularly when in segmental patterns, are truly clonal.26,27
The clinical history is one of the most important keys to diagnosing an IH (Box 126-1).28 Absence at birth or presence as a premonitory mark, usually an area of pallor, telangiectasias, or duskiness is characteristic, whereas a fully formed soft-tissue mass at the time of birth is most likely not an IH, but another vascular anomaly or other disease process.
|
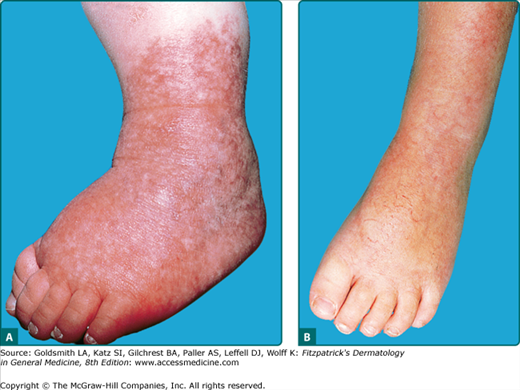
Though IH are not present at birth as fully formed tumors, superficial IH almost always become apparent within the first month of life. The period of most rapid growth typically occurs within the first 5 months of life, with 80% of growth being completed by 5 months of age.29 Deep hemangiomas may be noted at a somewhat later age, on average 1 month later than superficial IH and uncommonly are not appreciated until a few months of life. Large, segmental, deep, and parotid gland hemangiomas may continue to enlarge slowly for months to years longer.30 This growth phase is followed by a slower involution phase which is more variable in length, lasting for months to years (Fig. 126-1). Evidence of involution (change to a dull red, then gray or milky-white color, followed by flattening and softening) is usually apparent by 1 year of age.29 Smaller hemangiomas typically involute sooner than very large ones, but there are exceptions. Most IH complete their course by the age of 7–10 years. Some children have normal skin after involution whereas the remainder has telangiectasias, atrophy, fibrofatty residuum, or scarring.
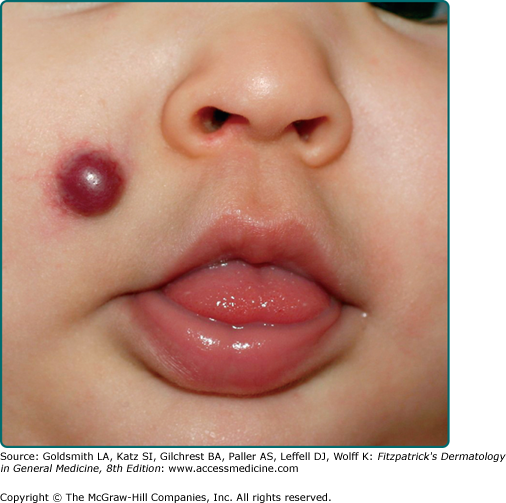
Hemangiomas which involve the upper dermis (so-called superficial hemangiomas) have a bright strawberry red color; whereas those with deep dermal and subcutaneous location are skin color to blue in color. Those involving both superficial and deeper skin structures, so-called mixed hemangiomas, have both features. In addition to this clinical appearance, IH can be classified as localized, segmental, or indeterminate.31,32 Localized hemangiomas exhibit clear spatial containment as if arising from one central focus (Fig. 126-2). Segmental hemangiomas (similar to other segmental dermatologic disease such as vitiligo and neurofibromatosis) correspond to a portion of a developmental segment or broad anatomic territory (Fig. 126-3).33 They are often plaque-like in nature with a linear or geographic configuration. Those hemangiomas not clearly identifiable as localized or segmental are termed indeterminate (see Fig. 126-3). The patterns of facial segmental IH have been found to correspond to neural-crest-derived facial prominences, and a new map for them has recently been proposed.14 Multifocal hemangiomas are usually multiple localized hemangiomas. Though the exact number is somewhat arbitrary, the presence of more than five hemangiomas confers a risk of extracutaneous hemangiomas.
Classification of hemangiomas by subtype not only facilitates communication but also helps predict risk of complications and need for treatment. A prospective study of 1,058 children with IH showed that segmental hemangiomas are 11 times more likely to experience complications and eight times more likely to receive treatment than localized hemangiomas even when controlled for size.34
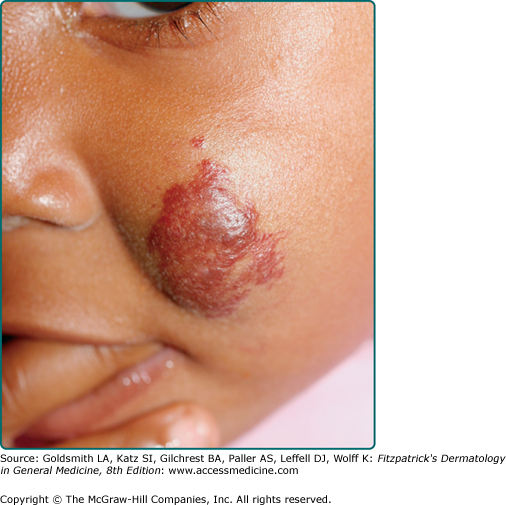
Atypical presentations of IH include deep hemangiomas and those with minimal to no proliferation. Deep IH proliferate in the lower dermis and subcutaneous tissue without penetration of the papillary dermis (Fig. 126-4). They present as a localized, firm, rubbery subcutaneous mass that can be slightly raised with a bluish color or with telangiectasias involving the overlying skin, or they may be deep enough that the overlying skin is completely flat and of normal hue. If there is involvement of the papillary dermis, the cutaneous surface is often bright red and thus classified as a superficial and deep IH. IH with minimal to absent proliferation often have fine telangiectasias with a minimal to absent proliferative component thus resembling persistent premonitory IH (Fig. 126-5). They have recently been shown to be GLUT-1 positive indicating that they are indeed IH, but for unknown reasons, have not had a rapid proliferative phase. For unknown reasons, they are more common in the lower body.35,36
Certain hemangiomas have known associated risks. Anatomic location is one of the most important factors affecting risk. Hemangiomas involving the central face (including the nose and perioral skin), periocular area, neck, mandibular region, and perineum should alert clinicians to possible increased risk of complications. In addition, the presence of multiple or segmental hemangiomas is associated with greater risk of extracutaneous disease.28
Facial segmental IH are associated with PHACE (Online Mendelian Inheritance in Man #606519), a neurocutaneous syndrome that consists of the following features: posterior fossa brain malformations, segmental cervicofacial hemangioma, arterial anomalies, cardiac defects or coarctation of the aorta, eye anomalies, and sternal defects, such as sternal clefting or supraumbilical raphe.37 A recent workshop reviewed knowledge about PHACE and consensus criteria for diagnosis have been proposed (see Table 126-1).38,39 Up to one third of patients with large facial hemangiomas will be found to have PHACE when studied thoroughly.38 While initial reports emphasized structural brain abnormalities, particularly Dandy–Walker malformation, recent reports have found that arterial anomalies of the head and neck are more common than structural brain abnormalities.37–40 These intracranial arterial defects can lead to a Moyamoya phenomenon, ischemia, and stroke.41,42 Other complications including seizure disorders and developmental delay are sometimes present in the setting of these CNS abnormalities.40 The most common cardiac anomaly is coarctation of the aorta, most often involving the transverse aorta. Brain imaging [magnetic resonance imaging (MRI) and magnetic resonance angiography] should be considered in all infants with a large segmental hemangioma of the face.43 In addition, formal ophthalmologic examination and echocardiogram may be performed, given the relative frequency of anomalies in these sites. Periodic developmental and neurologic assessments should be performed.
PHACE Syndrome Facial hemangioma >5 cm in diameter PLUS 1 major criterion OR 2 minor criteria | ||
Possible PHACE Syndrome | ||
Facial hemangioma >5 cm in diameter PLUS 1 minor criterion | Hemangioma of the neck or upper torso PLUS 1 major criterion OR 2 minor criteria | No hemangioma PLUS 2 major criteria |
Organ System | Major Criteria | Minor Criteria |
| Cerebrovascular | Anomaly of major cerebral arteries | Persistent embryonic artery other than trigeminal artery |
| Dysplasiaa of the large cerebral arteriesb | Proatlantal intersegmental artery (types 1 and 2) | |
| Arterial stenosis or occlusion with or without moyamoya collaterals | Primitive hypoglossal artery | |
| Absence or moderate to severe hypoplasia of the large cerebral arteries | Primitive otic artery | |
| Aberrant origin or course of the large cerebral arteriesb | ||
| Persistent trigeminal artery | ||
| Saccular aneurysms of any cerebral arteries | ||
| Structural brain | Posterior fossa anomaly | Enhancing extra-axial lesion with features consistent with intracranial hemangioma |
| Dandy-Walker complex or unilateral/bilateral cerebellar hypoplasia/dysplasia | Midline anomalyc | |
| Neuronal migration disorderd | ||
| Cardiovascular | Aortic arch anomaly | Ventricular septal defect |
| Coarctation of aorta dysplasiaa | Right aortic arch (double aortic arch) | |
| Aneurysm | Aberrant origin of the subclavian artery with or without a vascular ring | |
| Ocular | Posterior segment abnormality | Anterior segment abnormality |
| Persistent fetal vasculature (persistent hyperplastic primary vitreous) | Sclerocornea | |
| Retinal vascular anomalies | Cataract | |
| Morning glory disc anomaly optic nerve hypoplasia | Coloboma | |
| Peripapillary staphyloma | Microphthalmia | |
| Coloboma | ||
| Ventral or midline | Sternal defect | Hypopituitarism |
| Sternal cleft | Ectopic thyroid | |
| Supraumbilical raphe | ||
| Sternal defects | ||
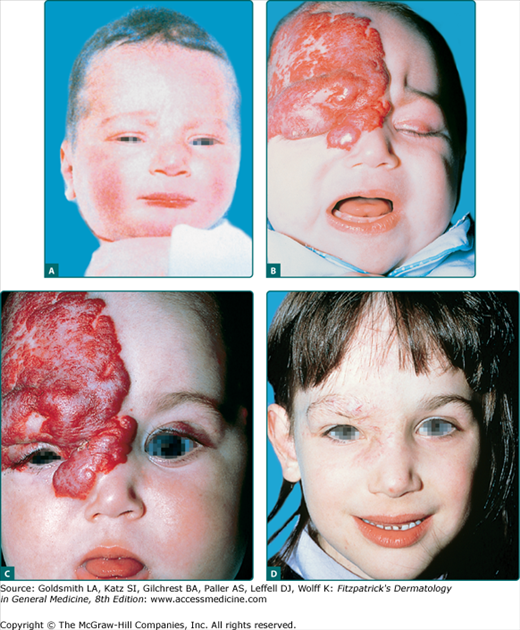
Infants with periocular hemangiomas are at risk for anisometropia and amblyopia, which if untreated, can lead to permanent visual loss (Fig. 126-6).44,45 Direct pressure on the cornea can produce astigmatism or myopia, and the mass effect of the tumor itself can cause ptosis, proptosis, visual axis occlusion, or strabismus. Any patient with a hemangioma in the periocular area should have a prompt formal ophthalmologic evaluation with repeat visits during the proliferative phase (typically the first 3–4 months of life). Imaging studies may be needed to assess whether retrobulbar involvement is present.
Figure 126-6
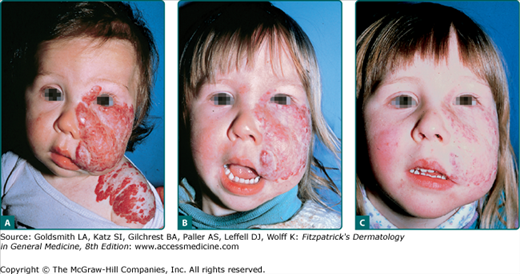
Vision-endangering segmental infantile hemangioma treated with systemic glucocorticoids. A. No premonitory signs of tumor seen on infant’s photograph. B. By age 3 months, extensive infantile hemangioma infiltrating the upper lid and surrounding tissue, causing blocked vision. C. Eyelid opened within 2 weeks of glucocorticoid therapy. D. Involuted tumor, age 6 years.
Segmental hemangiomas involving the preauricular, mandibular, chin, and neck skin (or so-called beard area) have a 60% risk of having symptomatic airway disease.46 Airway hemangiomas often present with the insidious onset of biphasic stridor between weeks 4 and 12 of life and are often mistakenly diagnosed as tracheomalacia, upper respiratory infection, or croup. If the hemangioma continues to enlarge, respiratory distress can ensue and become life-threatening. Prompt evaluation by a pediatric otolaryngologist and treatment is essential.47 Hemangiomas can also involve the parotid gland, and may require treatment due to massive growth, deformity of adjacent structures, and, in rare cases, high-output congestive heart failure.48,49
Segmental hemangiomas overlying the lumbosacral or perineal area can have associated spinal, bony, and genitourinary anomalies. Two acronyms have been proposed for this constellation of findings: PELVIS syndrome for perineal hemangioma, external genitalia malformations, lipomyelomeningocele, vesicorenal abnormalities, imperforate anus, and skin tag, and SACRAL syndrome, denoting spinal dysraphism, anogenital anomalies, cutaneous anomalies, renal and urologic anomalies, associated with angioma of lumbosacral localization.50,51 Segmental hemangiomas overlying the lumbosacral spine have a significant risk of spinal dysraphism and tethered spinal cord. MRI is strongly recommended in this setting.52 Other evaluations such as renal ultrasound should be based on clinical findings.
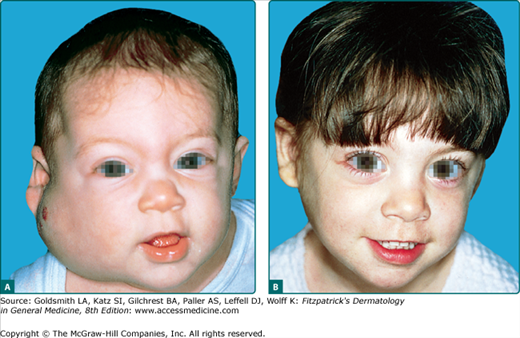
Approximately 15% of infants will have more than one hemangioma, and premature infants have an even higher risk of multiple lesions (Fig. 126-7). In rare cases, infants can have hundreds of lesions. Until Glut-1 staining was recognized as a specific marker for IH, many infants with multifocal vascular tumors and extracutaneous disease were described as having diffuse neonatal hemangiomatosis. Many of these infants however actually have other vascular tumors. Infants with 5 or more IH are known to have an increased risk of having hepatic hemangiomas. Other sites of visceral involvement are exceeding rare in true IH. Visceral hemangiomas including those affecting the liver, gastrointestinal tract, and brain, have also been reported with solitary segmental hemangiomas.53
The liver is the most common extracutaneous site of IH, Infants with >5 IH should be evaluated for the possibility of liver hemangiomas with liver ultrasound.54 Even if present, hepatic hemangiomas are often asymptomatic, however a minority cause morbidity, and in rare cases are life threatening. A classification for liver hemangiomas has been proposed by Christison-Lagay et al including three types of hepatic hemangiomas, two of which are true IH.55 The most common type (the presence of a few or multifocal liver hemangiomas) is often asymptomatic but can cause high-output congestive heart failure. A much rarer condition where the liver is virtually replaced by hemangiomas is termed “diffuse” disease. This life-threatening condition can result in abdominal compartment syndrome and a severe form of hypothyroidism due to tumor-related deiodination of thyroid hormone (see Section “Laboratory Tests”). A third type of hepatic hemangioma, wherein large solitary vascular tumors are typically present at birth is associated with arteriovenous shunting. In most cases these are not true infantile hemangioma but more likely analogous to rapidly involuting congenital hemangioma (RICH) occurring in the liver.
When cardiac compromise or severe hypothyroidism is a complication of hepatic hemangioma, systemic intervention is necessary. Embolization may be helpful if high-output congestive heart failure is present.56,57 Aggressive thyroid hormone replacement is needed in cases with hypothyroidism.58 In life-threatening cases liver transplant may be considered as a therapeutic option.
Hypothyroidism is a rare complication in infants with massive hemangiomas of the liver.59,60 First reported by Huang et al, hemangioma tissue demonstrates high levels of type 3 iodothyronine deiodinase activity, which accelerates the degradation of thyroid hormone.58,61 Infants with significant hepatic hemangiomas should have thyroid function evaluated, including T3 (the hormone consumed) and thyroid-stimulating hormone, because T4 levels may initially remain normal. Conversely, screening liver ultrasound looking for hemangiomas should be performed in infants with hypothyroidism of unknown etiology even in the absence of cutaneous hemangiomas. Hypothyroidism and other endocrine abnormalities have also been reported with PHACE syndrome. Infants with PHACE syndrome, large cutaneous hemangiomas, or extensive hepatic haemangiomas should have thyroid function [specifically thyroid-stimulating hormone, triiodothyronine (T3), and thyroxine (T4)] checked routinely.62
Platelet studies are not indicated in IH as the Kasabach-Merritt phenomenon (KMP) is not associated with IH but rather with kaposiform hemangioendothelioma (KHE) and tufted angioma (TA) (see Section “Pharmacologic Therapy”).
Recommended tests and imaging studies are included in Section “Cutaneous Lesions” with discussion on specific IH and their related physical findings.
Ulceration is the most common complication of IH, occurring in approximately 15% of patients usually during the proliferative phase with a median age of onset of 4 months.63 It occurs most frequently with segmental IH and at sites that are exposed to moisture and friction such as the perioral, perianal, and other intertriginous sites.64 Secondary infection can occur but its frequency is debatable. Cultures usually show polymicrobial growth and are likely the result of colonization.65 Localized infection will often improve with the use of topical mupirocin or metronidazole. However, if deeper or persistent infection is suspected, systemic antibiotics should be prescribed.
Local wound care, barrier protection, and pain control are essential for treatment. Bio-occlusive dressings may be helpful but those intended to stick to the skin may be limited by location because they do not adhere well near orifices.66,67 In these areas, thick applications of petrolatum-based ointments can be helpful. Pain can be a major issue in management. It can be minimized with an occlusive dressing, oral acetaminophen with or without codeine, and the use of very small amounts of topical lidocaine ointment no more than a few times a day.64 Ulcerations generally heal with scarring within 2–3 weeks with topical care. For refractory cases, other treatment modalities including pulsed dye laser (PDL) or Becaplermin 0.01% gel, a synthetic platelet-derived growth factor, have anecdotally been reported to be effective.68,69 Therapies aimed at halting hemangioma growth, such as intralesional and systemic steroids or excision, may be useful in some cases.
Other serious complications such as hypothyroidism, internal organ involvement, or vital structure compromise due to the location of IH in certain anatomic sites have been discussed in Section “Cutaneous Lesions.”
The prognosis of most IH is excellent, with spontaneous involution and little to no sequelae, but a significant minority of IH result in permanent disfigurement or medical sequelae. Certain characteristics are associated with an increased risk of complications and need for treatment (Table 126-2).29,70 Consideration of early treatment should be given to hemangiomas with these characteristics, depending on the specific clinical setting.
Anatomic Location/Morphology | Associated Risk |
|---|---|
Facial, large segmental | PHACE syndrome (posterior fossa malformations, hemangiomas, arterial anomalies, cardiac defects, eye abnormalities, sternal clefting) |
Nasal tip, ear, large facial (especially with prominent dermal component) | Permanent scarring, disfigurement |
Periorbital and retrobulbar | Ocular axis occlusion, astigmatism, amblyopia, tear-duct occlusion |
Segmental “beard area,” central neck | Airway hemangioma |
Perioral | Ulceration, disfigurement, feeding difficulties |
Segmental overlying lumbosacral spine | Tethered spinal cord, genitourinary anomalies |
Perineal, axilla, neck, perioral | Ulceration |
Multiple hemangiomas | Visceral involvement (especially liver, gastrointestinal tract) |
The decision to initiate treatment is based on many factors, including size and location, psychosocial implications, and risks and benefits of the proposed therapy. For the majority of small hemangiomas, close observation and follow up is the most appropriate approach. This does not mean doing nothing. The infant should be seen frequently, especially during the first few months (corresponding to the proliferative phase). During these visits, education about the natural course of IH and discussions about the psychosocial impact on the child and/or the family should occur.71 Photographs of the likely outcome for a similar lesion are often helpful. Many parents experience anxiety and may find themselves subject to comments from complete strangers about their child’s hemangioma.72 Most parents of young children do not think their child is deeply affected by these reactions, but facial hemangiomas, in particular, can cause psychological suffering once the child reaches school age.73,74 Potential treatment options should be discussed well ahead of entrance to elementary school.
If it is decided that treatment is necessary, options include pharmacologic, laser, or surgical interventions (see Table 126-3). However, management remains controversial with few double-blind controlled studies and no Food and Drug Administration-approved labeled indications for medical treatments.
Treatment | Major Side Effects |
|---|---|
Systemic corticosteroids | Diminished gain of height and weight, cushingoid facies, personality changes, gastric irritation, hypertension, immunosuppression |
Intralesional corticosteroids | Arterial embolization/injection, atrophy, systemic absorption |
Topical corticosteroids | Local atrophy, systemic absorption |
Propranolol | Hypotension, symptomatic bradycardia, hypoglycemia, agitation, sleep alteration, sweating, cold hands, wheezing |
Interferon-α | Spastic diplegia (neurotoxicity), fever, hepatotoxicity, neutropenia, anemia |
Vincristine | Central line placement, neuropathy, abdominal pain, constipation |
Laser | Pain, scarring, hypopigmentation, textural change, ulceration |
Surgery | General anesthesia, scarring |
Until recently systemic corticosteroids were the first-line treatment for deforming, endangering, or life-threatening IH (see Fig. 126-6).75 They work best during the growth phase, causing slowing or cessation of growth in up to 90% of cases, with actual shrinkage in approximately one third. Although the mechanism of action is not well understood, recent studies suggest the upregulation of mitochondrial cytochrome b, clusterin/ApoJ (possible apoptotic markers), and/or interleukin-6 as markers of corticosteroid-induced cessation of hemangioma growth.76–78 Prednisone or prednisolone is given at a dose of 2–3 mg/kg/day, typically for 4–8 weeks followed by a tapering of varying length, depending on the age of the child and indication for treatment. A meta-analysis showed an 84% response rate with an average dose of 2.9 mg/kg for a mean of 1.8 months before tapering. Although 3 mg/kg/day is more effective (94% response) than 2 mg/kg/day (75% response), greater adverse events are found with the higher dose.79
Short-term complications of systemic corticosteroids include: cushingoid faces (71%), personality changes (29%), gastric irritation (21%), fungal infection (oral or perineal, 6%), and diminished gain of height (35%) and weight (42%) during treatment. More than 90% of children with diminished gain of height return to their pretreatment growth curve by 24 months of age (Table 126-3).80,81 Other complications include hypertension, steroid-induced myopathy, immunosuppression, and transient adrenal insufficiency.81,82 Blood pressure should be monitored with each visit to the dermatologist or pediatrician.83 Children taking more than 2 mg/kg/day of prednisone for longer than 14 days are considered to have a deficit in cell-mediated immunity. Live viral vaccinations should be deferred in infants receiving high-dose corticosteroids. Rare cases of Pneumocystis carinii pneumonia have been reported in this setting, leading some physicians to use trimethoprim-sulfamethoxazole prophylaxis during treatment.84,85
Intralesional corticosteroids can be an effective treatment for relatively small localized hemangiomas located in high-risk sites such as the lip, nasal tip, cheek, and ear. Injections for periocular hemangiomas are usually performed by ophthalmologists, but reports of retinal artery embolization and blindness have resulted in a reduced use of this modality.86–87 The largest published case series of intralesional steroids found that the majority showed greater than 50% reduction in volume with the best results occurring in relatively superficial hemangiomas. Adverse events occurred in 6.4% of patients and included cushingoid appearance, cutaneous atrophy, and anaphylactic shock (see Table 126-3).88
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree