Transplantation of Pediatric En Bloc Kidneys
Christoph Troppmann
Richard V. Perez
DEFINITION
Pediatric en bloc kidney transplantation is defined as the transplantation of both kidneys from a pediatric deceased donor into one recipient as a single anatomic unit (i.e., leaving the kidneys attached to the donor’s abdominal aorta and inferior vena cava [IVC]). En bloc kidney donors typically weigh between 2.5 and 25 kg. The goal of this surgical approach is to provide the usually adult recipients of en bloc grafts with sufficient graft nephron mass while minimizing the technical challenges and complication rates that would result from transplanting these small kidneys as single grafts.
The surgical considerations and techniques described in this chapter focus on transplantation of en bloc kidneys from smaller (<10 kg) and very small (<5 kg) pediatric donors. These principles are, however, equally relevant for the transplantation of en bloc kidneys from larger (>10 kg) pediatric donors.
SURGICAL MANAGEMENT
Many kidneys from donors that weigh between 15 and 25 kg are transplanted as single grafts.1,2,3,4,5,6 Depending on donor history and graft characteristics, however, kidneys from selected donors in the 15- to 25-kg weight range may still benefit from being transplanted en bloc (vs. as single kidneys) in order to address any anatomic contraindications to splitting (e.g., multiple renal arteries) and potential functional challenges resulting, for instance, from significant preterminal acute kidney injury, donation after cardiac death (DCD) donor status, and long warm and cold ischemic times.
En bloc kidney grafts are low-flow systems due to organ size and are thus intrinsically at higher risk for graft thrombosis.1,7 Optimal recovery and transplant techniques are therefore absolute prerequisites for good recipient outcomes.8,9,10
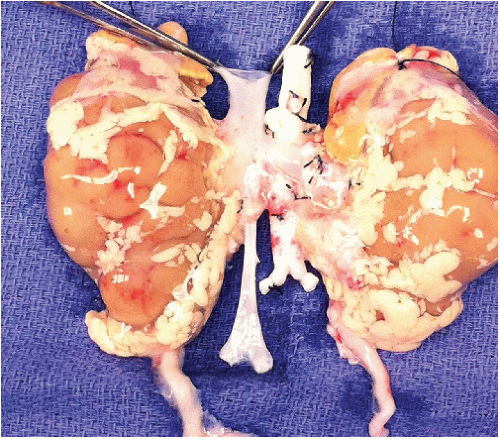
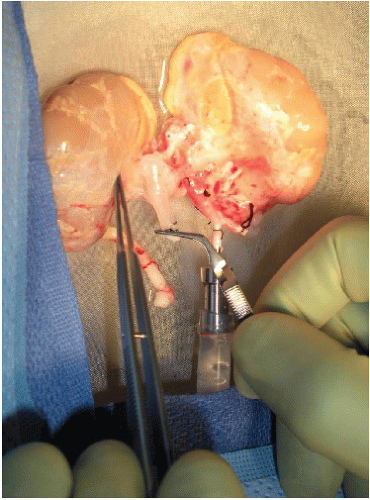
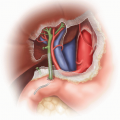
Ensure that the en bloc graft was appropriately recovered (FIG 1).
Follow the specific recovery guidelines outlined in Chapter 2 (Procurement of Pediatric En Bloc Kidneys).
If the en bloc graft is to be imported, share the aforementioned specific surgical recovery guidelines for pediatric en bloc kidneys with the outside recovering team prior to the start of brain dead and DCD donor recoveries, particularly for smaller (<10 kg) donors. Emphasize the need for sufficient suprarenal vascular cuff length and for inclusion of the aortic and caval bifurcations, and the strong preference for the use of University of Wisconsin (UW) preservation solution (SPS-1®; Organ Recovery Systems, Inc, Itasca, IL).11,12,13
Donor and graft selection
Donor characteristics
Weight greater than or equal to 2.5 kg
Brain-dead and DCD donors14
Preterminal acute kidney injury is acceptable,9 but preterminal anuria in a donor less than 15 kg constitutes an absolute contraindication.
Donor risk factors for perioperative complications, including graft thrombosis and primary nonfunction, include weight less than 5 kg,7 acute kidney injury, DCD donor status, and long DCD warm ischemic time (e.g., >60 minutes).
Graft and preservation risk factors for perioperative complications (including graft thrombosis and primary nonfunction)
Multiple renal arteries on one or both kidneys
Use of a solution for flush and preservation other than UW solution (i.e., histidine-tryptophan-ketoglutarate [HTK] solution [Custodiol® HTK, Essential Pharmaceuticals, Newtown, PA]).11,12,13
Extended preservation time (e.g., >36 hours)7
Low flow and high resistance while on hypothermic pulsatile perfusion (see the following text for donor weight-specific details)
Recipient selection
Weight is the most commonly used variable to gauge donor kidney size and functional reserve. Similarly, a specific
recipient’s metabolic needs with respect to graft nephron mass are clinically most commonly estimated by using the recipient’s weight. Donor and recipient weight are thus important criteria for matching a recipient to a specific en bloc graft.
Ideal recipient weight for donors less than 5 kg is less than 60 kg; for donors 5 to 10 kg, less than 75 kg; for donors 11 to 20 kg, less than 85 kg.
Age: 16 to 75 years
For pediatric recipients younger than 16 years old, a kidney from a standard criteria, adult deceased donor is preferred due to more predictable immediate function and better functional reserve.
Older recipients are adequate if they are not frail and have sufficient cardiac reserve (see below).
Relative recipient contraindications
Primary renal disease with potential for significant recurrence
Increased immunologic risk (e.g., presence of donor-specific antibody)
Excessive recipient size and weight. This would not only create a functional mismatch between graft and recipient but might also require a deeper pelvic dissection, resulting in suboptimal exposure and mobilization of the recipient’s iliac artery and vein. Moreover, a heavier recipient’s peritoneal sac overlying the en bloc graft may cause vascular compromise by direct compression of the graft’s renal veins and IVC.
Recipient frailty—the recipient must be able to with-stand peritransplant volume loading for optimization of systemic hemodynamics and graft perfusion, surgical stress, a possible relaparotomy, as well as a potentially prolonged posttransplant dialysis period of up to several months until kidneys have sufficiently grown.
Absolute recipient contraindications
Anuria. Nonused bladders are likely to be very small and could not be reached without placing the en bloc graft’s short ureters under considerable tension.
Significant bladder pathology and major prior bladder operations. A significant amount of perivesical and vesical fibrosis may not only prevent adequate bladder distension and bladder mobilization (to avoid tension on the graft ureters) but also constitutes a risk factor for early postoperative urine leaks.
Significant history of thrombotic events or a defined hypercoagulable state (e.g., factor V Leiden mutation, deficiency of protein C and protein S)
Prior transplants into the right and left iliac fossa (resulting in limited implantation and graft positioning options due to prior dissection and use of both external iliac arteries and veins)
The final decision to accept a specific en bloc graft for transplantation must always be based on careful consideration of all of the aforementioned donor, graft, preservation, and recipient risk factors’ additive effects.7,8,9,10,14
Recipients should specifically give consent for a pediatric en bloc transplant. Recipients of grafts from smaller (<10 kg) donors must be informed that long-term outcomes for technically successful pediatric en bloc transplants are equivalent or even superior to the outcomes of live donor transplants but that these outcomes are achieved at the expense of slightly higher early complication and reintervention rates.9,10,15,16,17
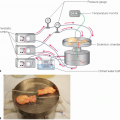
Hypothermic pulsatile perfusion
Preimplantation machine perfusion of pediatric en bloc kidneys is an important prerequisite for achieving good outcomes, particularly for grafts from smaller (<10 kg) donors, DCD donors, and donors with acute kidney injury.7,8,9,10,14,18,19,20
Benefits of hypothermic pulsatile perfusion
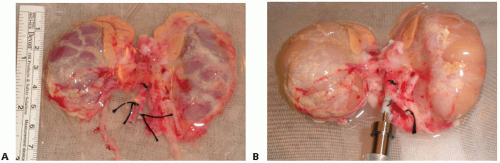
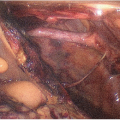
Provides a complete flush for kidneys that had a poor in situ flush, thus enhancing preservation quality. Kidneys from very small donors often flush suboptimally (likely due to anatomically very small renal arteries, particularly if there are multiple renal arteries; preterminal renal vasoconstriction in the donor; and aortic flush cannula malposition with the cannula tip being advanced beyond the renal artery orifices, which can occur easily with the short abdominal aorta of smaller donors) (FIG 2A,B).
Allows to assess quality of vascular repairs and reconstructions and of the graft’s back-table preparation (including ascertaining absence of renal vein outflow compromise, following closure of the proximal IVC and absence of renal artery takeoff narrowing, following closure of the suprarenal aorta with an aortorenal aortic lid as well as identification of leaking vascular structures in the interrenal central tissues anterior and posterior to the IVC and aorta)18,21
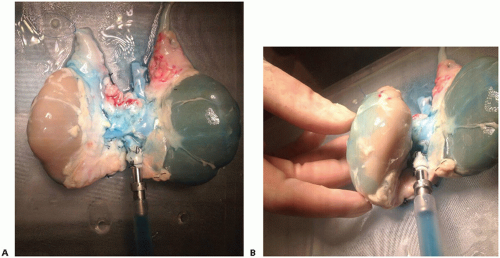
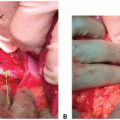
Provides the opportunity to assess integrity of arterial perfusion (if necessary) by injection of 1 to 2 mL of methylene
blue (methylene blue injection, USP [10 mg/mL]) into the perfusion circuit (FIG 3A,B)
Helps to identify kidneys at increased risk for early posttransplant graft loss and primary nonfunction based on pump flow and resistance (absolute values and trends) and provides objective data to help determine whether a graft should be discarded18,19,20
May decrease risk of early posttransplant graft thrombosis for en bloc grafts from very small donors by decreasing resistance and increasing flow
Improves preservation quality (as compared to static cold storage) for kidneys that require prolonged preservation22
Improves early graft function, thus facilitating clinical early posttransplant management
Machine perfusion was associated—independently of donor and recipient characteristics—with higher graft survival rates (for adult deceased donor kidneys) in a recently published large international, randomized controlled multicenter trial.22
TECHNIQUES
BACK-TABLE BENCH PREPARATION: PRINCIPLES
The back-table sections describe the standard en bloc graft back-table preparation (i.e., preparation of the distal donor aorta and IVC for end-to-side anastomosis to the respective recipient vessels) as well as surgical-technical options to manage suboptimally recovered but potentially still salvageable en bloc grafts.
Use surgical loupes for the back-table preparation (magnification 2.5× to 3.5×).
The graft preparation is done on the back table with the kidney graft placed on ice slush. After complete preparation of the donor aorta, including surgical closure of the suprarenal aorta, as necessary, the back-table preparation can be completed on the pulsatile perfusion pump, depending on surgeon preference and time constraints. The perfusate distends the renal veins and IVC and significantly expedites their preparation. The venous distension facilitates identification of lumbar veins and other venous tributaries.18
The most important guiding surgical principle for the back-table preparation is the necessity for extremely gentle tissue handling. The smaller the donor, the more important this principle becomes.
Avoid exerting traction on the renal vascular pedicles under all circumstances. Do not lift the en bloc graft by grasping one of the two kidneys as a handle (and thus placing traction on both renal vascular pedicles).
The delicate ureters and periureteral tissues warrant careful and atraumatic handling to minimize the risk for posttransplant ureteral complications (e.g., ischemic strictures, leaks).
Minimize dissection of the central periaortic and pericaval tissues and structures. Do not try to identify and ligate the proximal (perihilar) segment of the left gonadal vein.
Minimize dissection of the perinephric fat. In infant and small pediatric donors, the perinephric fat outside and inside Gerota’s fascia is usually only, very sparsely developed (FIG 2A). Excessive, overzealous dissection of the perinephric fat may only cause injury to extrarenal
branches of the renal artery and to hilar structures without yielding any benefit. If necessary, remove only the nonhilar perinephric fat to assess flush quality, to perform a visual inspection, and to facilitate observation of the kidney surface while on pump (FIGS 2 and 3).
Leave both adrenal glands attached to the kidneys until after graft reperfusion when they are excised (FIG 1). Ligate and divide the left and right adrenal vein in close proximity to each adrenal gland. Carefully ligate and divide the tissues connecting the inferomedial aspect of each adrenal gland to the hilar structures. The adrenal glands can now advantageously be used as handles to avoid grasping the kidneys themselves. Tie each end of a long 4-0 silk to the adrenal glands’ upper pole, creating effectively a bucket handle-like structure that can be used for atraumatic lifting of the kidneys as they are being placed on pump, for manipulation while on pump, and at the time of the implantation.
Use 4-0 and 5-0 silk ties when tying off vascular structures and tissues.
INITIAL BACK-TABLE GRAFT ASSESSMENT AND PREPARATION
Assess flush quality (FIG 2).
Inspect the kidney surfaces (e.g., for petechial lesions, infarcts).
Assess kidney size and extent of the dissection of the central interrenal tissues anterior and posterior to the aorta and the IVC.
Assess vascular structures.
Determine vascular cuff length of the proximal donor aorta and IVC and whether the distal aortic and caval bifurcations were included in continuity with aorta and IVC, respectively (FIG 1,4).
Any direct injury to a renal artery and to a renal vein, including a partial or complete detachment of a renal vein from the donor IVC, constitutes an absolute contraindication to the use of the en bloc graft (repairs of direct arterial and venous injuries other than those that can be addressed by inclusion into an aortic or caval lid closure are associated with prohibitively high thrombosis rates).
Inspect any arterial donor vessels sent with the en bloc graft.
Place the kidneys in an anatomic position and orientation, facing the surgeon. Carefully dissect the distal donor aorta and aortic bifurcation as well as any attached iliac artery segments. Create a branch patch on the distal donor aorta at this stage only if only a minimal part of the aortic bifurcation was recovered in continuity with the donor aorta. Plan to insert the pump cannula directly into the aorta under those circumstances. If both common iliac arteries were recovered in continuity with the donor aorta (as would ideally be the case), ligate one of the two common iliac arteries. Use the contralateral common iliac artery for insertion of the pump cannula.18
Ligate the inferior mesenteric artery.
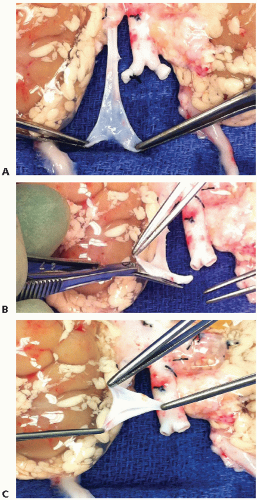
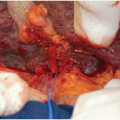
Carefully dissect the distal donor IVC and ligate the right gonadal vein in the vicinity of the IVC. Create an IVC branch patch if the caval bifurcation was recovered in continuity with the IVC (FIG 4A-C).
Flip the kidney graft onto its anterior aspect. Identify and carefully ligate all posterior lumbar branches of the aorta and IVC with 5-0 silk ties, starting inferiorly and moving to just below the level of the retrocaval segment of the right renal artery. The right renal artery should never be directly identified. Leave it encased by the surrounding
retrocaval and other retroperitoneal tissues to avoid direct renal arterial injury. Be mindful during this phase of the preparation for the potential presence of a retroaortic left renal vein. Tie off all remaining lumbar branches of the aorta and IVC above the level of the right renal artery. During the identification and ligation of the lumbar arteries on the aorta, it is paramount to remain strictly in the midline, avoiding the posterolateral aspects of the aorta (where the main and smaller accessory renal arteries may originate). This approach helps preventing inadvertent injury to, or ligation of, the renal arteries.
Particularly for en bloc grafts from very small (<5 kg) donors, it is preferable to complete the preparation of the donor aorta first (including the closure of the suprarenal aorta as necessary). The venous back-table preparation can then advantageously be done when the graft is already on pump and the IVC is temporarily occluded proximally and distally (e.g., by using a small bulldog clamp) (FIG 5). The ensuing IVC distension can then significantly facilitate the identification and ligation of the lumbar veins and other venous IVC tributaries (FIG 5).
Flip the en bloc graft back onto its posterior aspect and rotate it 180 degrees in a coronal plane so that the proximal aorta and IVC face the surgeon. Dissect the suprarenal IVC cuff and identify the superior edges of the two renal vein confluences with the donor IVC.
SURGICAL BACK-TABLE MANAGEMENT OF THE SUPRARENAL INFERIOR VENA CAVA
Default management of the suprarenal IVC cuff is a primary transverse running closure with one row of running 7-0 polypropylene suture (Prolene®, Ethicon, Inc, Cincinnati, OH), taking very small bites and using as little as possible of the IVC cuff. Avoid sagittal closure of a short proximal IVC cuff as this technique may increase the risk for renal vein thrombosis. Sagittal closure places more traction and tension on both renal veins and can therefore more easily cause renal vein orifice distortion and outflow obstruction than a transverse closure.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree