Transaxillary Breast Augmentation
Louis L. Strock
DEFINITION
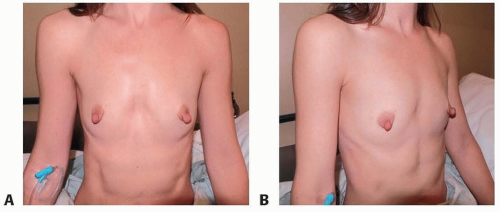
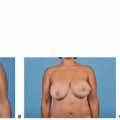
Hypomastia. This patient requested that she have a procedure to enlarge her breasts in a conservative way. She also stated that she preferred to have her breast implants placed in a way that would allow her to avoid incisions on her breasts (FIG 1A,B).
ANATOMY
To manage the request of this patient, the level and shape of the inframammary fold (IMF) will be lowered with the aid of endoscopic assistance. The pectoralis major muscle and overlying fascia will be divided according to external markings and correlated with internal muscle anatomy.
PATIENT HISTORY AND PHYSICAL FINDINGS
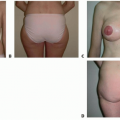
This patient is a 34-year-old woman who presented for breast augmentation after having had three children. She requested that her breasts be enlarged to a small C cup, with as soft a feel as possible. Her examination was remarkable for mild asymmetry, thin tissue, and large nipple size. Her breast base width measurement was 11 cm, and pinch thickness measurements were 1.5 cm laterally, superiorly, and medially. She was also noted to have extremely large nipples that she requested to have reduced at the time her breast implants were placed (FIG 2).
SURGICAL MANAGEMENT
Preoperative Planning
Preoperative planning centered on the choice of breast implant type in the context of her aesthetic goals and tissue type. She preferred the feel and intermediate projection of a Mentor MemoryGel smooth wall, round, moderate plus profile silicone gel device. Other options considered included the same device type in moderate and high profile versions, and a moderate height, moderate projection shaped highly cohesive gel device. She stated preference of a partial subpectoral plane of placement over a subfascial approach. Incision choices offered to this patient included inframammary and transaxillary, with the latter preferred by the patient to attempt to avoid incisions visible on her breasts. Nipple reduction was requested by the patient, to be performed following completion of the breast augmentation procedure and access incision closure.
Equipment
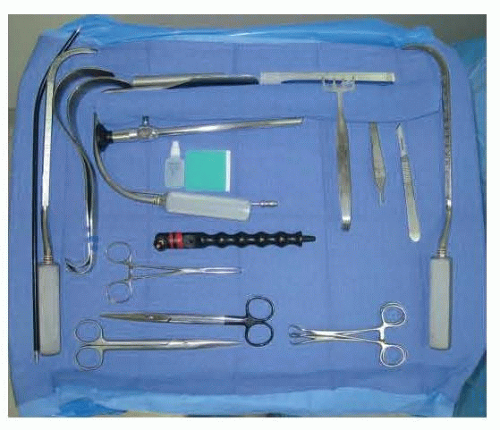
A standard HD endoscopic tower and camera are used in this procedure. This equipment is identical to that used for any subspecialty that utilizes an endoscopic tower and camera. The endoscope that is preferred is a 10-mm 30-degree angled scope, that is intended to fit correctly into the Emory Endoscopic retractor (FIG 3). A cautery handle with a suction end is used, and holds a cautery tube with a spatulated end. This is the basis for the dissection at the heart of this procedure. Additionally, 4-prong Freeman skin hooks, 2 mirror image Agris-Dingman dissectors, a 1-in fiberoptic retractor with suction port, facelift scissors, and two 1-in short Deaver retractors make up the instrument set for the procedure.1
Positioning
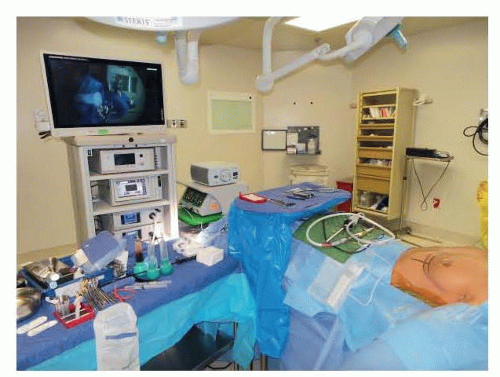
The patient is positioned with the arms out ninety degrees and straightened on armboards. All equipment, cords, and tubing are directed toward the feet of the patient in the midline. This allows for ease of transition during the procedure for device placement on either side. There is adequate
separation of the anesthesia equipment from the head and shoulders of the patient to allow the surgeon to stand above the shoulder on each side during the endoscopic tissue release portion of the procedure on each side (FIG 4).
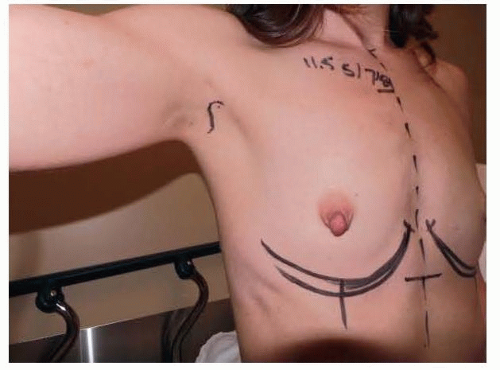
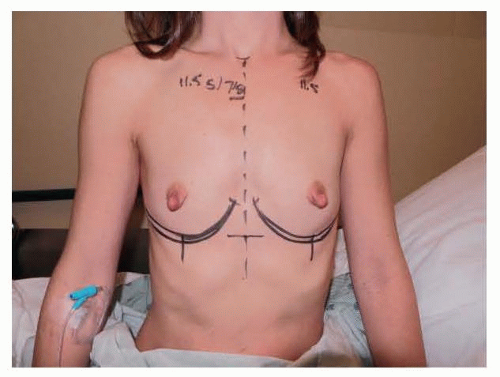 FIG 2 • Frontal markings show plan to lower the inframammary fold to accommodate dimensions of device to be used. |
Approach
The procedure can be performed adequately in this patient with use of inframammary or transaxillary approaches for incision access. The periareolar approach is more difficult given the relatively small size of the areola in this patient. Her thin tissue makes a partial subpectoral, or dual plane, approach preferred to maximize soft tissue cover over the implants.
TECHNIQUES
▪ Incision and Initial Dissection
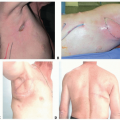
An S-shaped incision was planned, centered in the axillary apex (TECH FIG 1). This incision pattern was selected because it allows for a long functional length in a patient with a narrow area of hair-bearing skin in the axilla. The long portion of the incision was marked within the longest existing skin crease. The anterior extension is placed to stay behind the posterior aspect of the pectoralis major muscle. This is critical to keep the incision hidden during recovery. A cross-hatch is made centrally to facilitate skin closure. The incision is made through the hair-bearing skin to the subcutaneous tissue. The anterior skin flap is raised in an anterior direction toward the lateral edge of the pectoralis major muscle. The skin flap is kept thin to avoid entry into the axillary contents. This helps to avoid damage to the intercostobrachial nerve. Once the lateral border of the pectoralis major muscle is identified, its fascia is incised, and the subpectoral space is entered under direct vision. A finger sweep technique is used to further develop the separation between the pectoralis major and pectoralis minor muscles.
▪ Optical Cavity
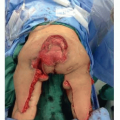
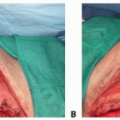
Once the entry between the incision and the space between the pectoralis major and minor muscles has been defined, the endoscopic retractor is introduced. Once correctly positioned, the 10-mm 30-degree-angled endoscope is brought into the operative field and placed into the retractor sheath. The camera head on the endoscope is checked for proper orientation, a critical step to ensure safety with the technique. The suction cautery is then used to create an optical cavity from the undersurface of the pectoralis major muscle (TECH FIG 2A). Staying on the undersurface of the muscle allows for variations in rib cage anatomy to not be a problem. This is performed in a uniform fashion to create optimal visualization of the pectoralis major muscle in preparation for the muscle release. The author feels that using the cautery to create the optical cavity is critical to avoid significant blood staining of tissues that can otherwise make endoscopic tissue dissection difficult. The key to this procedure is to avoid bleeding in the tissue pocket! (TECH FIG 2B-D). Though the initial descriptions of this procedure advocated use of the Agris-Dingman dissectors, the author has found that the occasional bloody outcome from that approach can be avoided with use of the cautery to create the optical cavity.2
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree