For the past 50 years, cemented total hip arthroplasty (THA) has been a highly successful surgical solution for end-stage hip disease.
 Although cementless fixation has grown in popularity and now dominates North American practice, cemented THA remains an evidence-based and appropriate treatment of hip pathology caused by a variety of degenerative, inflammatory, traumatic, vascular, developmental, and metabolic disorders.
Although cementless fixation has grown in popularity and now dominates North American practice, cemented THA remains an evidence-based and appropriate treatment of hip pathology caused by a variety of degenerative, inflammatory, traumatic, vascular, developmental, and metabolic disorders.
ANATOMY
 The hip is a diarthrodial synovial joint consisting of the articulation of the femoral head with the acetabulum. It functions as a ball-and-socket joint, with inherent bony constraints that define the range of motion. The laxity or tightness of the associated soft tissue also affects kinematics and function.
The hip is a diarthrodial synovial joint consisting of the articulation of the femoral head with the acetabulum. It functions as a ball-and-socket joint, with inherent bony constraints that define the range of motion. The laxity or tightness of the associated soft tissue also affects kinematics and function.
 The acetabulum develops at the junction between three embryologically distinct bones—the ilium, ischium, and pubis—which fuse at the triradiate cartilage during adolescence.
The acetabulum develops at the junction between three embryologically distinct bones—the ilium, ischium, and pubis—which fuse at the triradiate cartilage during adolescence.
 The acetabulum typically demonstrates 15 to 20 degrees of anteversion, as does the femoral neck. Normal combined anteversion is therefore 30 to 40 degrees, although the degree of anteversion varies considerably between individuals.
The acetabulum typically demonstrates 15 to 20 degrees of anteversion, as does the femoral neck. Normal combined anteversion is therefore 30 to 40 degrees, although the degree of anteversion varies considerably between individuals.
PATHOGENESIS
 Degenerative joint disease (DJD) is a final common pathway for various hip disorders of distinct etiologies.
Degenerative joint disease (DJD) is a final common pathway for various hip disorders of distinct etiologies.
Developmental abnormalities of the hip can lead to impingement and/or subluxation, abnormal joint reaction and articular shear forces, and consequent mechanical joint degeneration. These abnormalities include the following:
• Developmental dysplasia
• Coxa profunda
• Protrusio acetabuli
• Acetabular retroversion or pincer deformity of the acetabulum
• Pistol grip or cam deformity of the proximal femur
• Legg-Calvé-Perthes disease
• Slipped capital femoral epiphysis
Posttraumatic arthritis can develop after fractures of the femoral head, femoral neck, or acetabulum.
Osteoarthritis may also be idiopathic.
 Rheumatologic conditions such as rheumatoid arthritis and the seronegative spondyloarthropathies are caused by autoimmunity.
Rheumatologic conditions such as rheumatoid arthritis and the seronegative spondyloarthropathies are caused by autoimmunity.
 Osteonecrosis of the femoral head can result from many etiologic factors:
Osteonecrosis of the femoral head can result from many etiologic factors:
Alcoholism
Corticosteroid use
Chemotherapy
Sickle cell disease
Systemic lupus erythematosus
Vasculitis
HIV infection
Coagulopathy
 Osteonecrosis may also be idiopathic.
Osteonecrosis may also be idiopathic.
 Less commonly, metabolic disorders such as hemochromatosis and ochronosis, as well as hematologic abnormalities such as hemophilia and sickle cell disease, can cause advanced degeneration of the hip, as can rare congenital disorders, including epiphyseal and spondyloepiphyseal dysplasias.
Less commonly, metabolic disorders such as hemochromatosis and ochronosis, as well as hematologic abnormalities such as hemophilia and sickle cell disease, can cause advanced degeneration of the hip, as can rare congenital disorders, including epiphyseal and spondyloepiphyseal dysplasias.
NATURAL HISTORY
 The natural history of DJD is progression of disease. Although clinical symptoms may wax and wane, they generally become more severe, frequent, and debilitating over time.
The natural history of DJD is progression of disease. Although clinical symptoms may wax and wane, they generally become more severe, frequent, and debilitating over time.
Although medications can help control the progression of rheumatoid arthritis and other inflammatory conditions, no medical therapies currently have been proven to act as disease-modifying agents in DJD.
 When osteoarthritis is a consequence of anatomic abnormalities, there is hope that surgical correction of these abnormalities may unload the joint, halt progression of the disease, and even allow for biologic repair.
When osteoarthritis is a consequence of anatomic abnormalities, there is hope that surgical correction of these abnormalities may unload the joint, halt progression of the disease, and even allow for biologic repair.
Periacetabular osteotomy may positively impact the natural history of joint degeneration in acetabular dysplasia,25 but the long-term effect of osteotomy on hip function and progression of arthrosis is unknown. In the presence of moderate DJD, progression of the disease commonly occurs in spite of a well-performed osteotomy.
Similarly, osteochondroplasty of the femoral neck and acetabular rim may relieve symptoms associated with femoroacetabular impingement, but it is not known whether these procedures will reduce the progression of arthrosis.
PATIENT HISTORY AND PHYSICAL FINDINGS
 Initial evaluation should focus on identifying the extent to which hip pain can be attributed to intra-articular hip pathology. Hip pain may be localized to the groin, peritrochanteric region, thigh, knee, or, occasionally, below the knee. Lumbar spine disease may also cause pain in these regions.
Initial evaluation should focus on identifying the extent to which hip pain can be attributed to intra-articular hip pathology. Hip pain may be localized to the groin, peritrochanteric region, thigh, knee, or, occasionally, below the knee. Lumbar spine disease may also cause pain in these regions.
Although the source of the pain can usually be identified on the basis of physical examination, occasionally, selective anesthetic injection is helpful to elucidate the relative contributions of overlapping pathologies to a patient’s symptoms.
 Palpation is performed to assess for areas of tenderness, warmth, fluctuance, or mass.
Palpation is performed to assess for areas of tenderness, warmth, fluctuance, or mass.
Trochanteric bursitis is a common cause of hip area pain that can be ruled out by identifying a nontender bursa.
An inguinal mass may suggest that groin pain is related to a hernia.
 Active and passive range of motion should be assessed.
Active and passive range of motion should be assessed.
Flexion contracture commonly is encountered, as are limited internal rotation and abduction.
Limited external rotation, if present, impairs activities of daily living.
 Motor power of the abductors, adductors, flexors, and extensors is assessed and documented using a 5-point scale.
Motor power of the abductors, adductors, flexors, and extensors is assessed and documented using a 5-point scale.
Abductor weakness diminishes the likelihood of achieving a limp-free hip after arthroplasty.
 Gait should be assessed with the patient’s legs exposed and with and without use of walking aids.
Gait should be assessed with the patient’s legs exposed and with and without use of walking aids.
Trendelenburg gait suggests abductor weakness or hip discomfort.
Coxalgic gait suggests hip pain of any etiology.
Stiff hip gait may be present with hypertrophic osteoarthritis.
Short limb gait may be present with developmental dysplasia of the hip.
 Legs should be observed for leg length discrepancy (LLD). Some shortening is usually present in DJD. Severe shortening may be present in developmental dysplasia of the hip. Adduction contracture may cause apparent shortening, whereas abduction contracture may cause apparent lengthening. Pelvic tilt from spinal deformity may contribute to functional LLD. Pelvic tilt should be assessed in the standing and seated positions to determine whether it arises from LLD or spinal deformity.
Legs should be observed for leg length discrepancy (LLD). Some shortening is usually present in DJD. Severe shortening may be present in developmental dysplasia of the hip. Adduction contracture may cause apparent shortening, whereas abduction contracture may cause apparent lengthening. Pelvic tilt from spinal deformity may contribute to functional LLD. Pelvic tilt should be assessed in the standing and seated positions to determine whether it arises from LLD or spinal deformity.
 Examination of the spine should include inspection for deformity, palpation for tenderness, evaluation for pain with passive straight-leg raise, and a neurologic examination.
Examination of the spine should include inspection for deformity, palpation for tenderness, evaluation for pain with passive straight-leg raise, and a neurologic examination.
 Examination of distal pulses and capillary refill may reveal peripheral vascular disease that could be associated with vascular claudication.
Examination of distal pulses and capillary refill may reveal peripheral vascular disease that could be associated with vascular claudication.
 Tests of the hip include the following:
Tests of the hip include the following:
Thomas test: Inability to maintain extension of the ipsilateral hip reveals flexion contracture.
Patrick test: Discomfort with flexion, abduction, and external rotation of the hip suggests intra-articular hip pathology; however, this test may also provoke sacroiliac pain.
Ober test: Persistent abduction of the hip reveals tightness of the iliotibial band. This finding is important to note preoperatively so that it is not misinterpreted intraoperatively as overlengthening.
Impingement test: Hip pain with passive flexion, adduction, and internal rotation suggests intra-articular pathology but is not specific for femoroacetabular impingement.
Stinchfield test: Hip pain with resisted straight-leg raise suggests intra-articular pathology, typically affecting the central joint rather than the labrum.
Passive straight-leg raise: Radicular pain suggests lumbar pathology.
 Once pain has been localized to the hip, an assessment of pain, limp, extent of disability, and desired level of activity is warranted. This information allows the practitioner to give the patient a realistic assessment of the potential benefits of various therapeutic modalities.
Once pain has been localized to the hip, an assessment of pain, limp, extent of disability, and desired level of activity is warranted. This information allows the practitioner to give the patient a realistic assessment of the potential benefits of various therapeutic modalities.
 Skin over the affected hip should be assessed for mobility and the presence and location of scars from any prior surgical procedures, which may influence surgical approach.
Skin over the affected hip should be assessed for mobility and the presence and location of scars from any prior surgical procedures, which may influence surgical approach.
IMAGING AND OTHER DIAGNOSTIC STUDIES
 Plain radiographs should be obtained, weight bearing if possible.
Plain radiographs should be obtained, weight bearing if possible.
Low anteroposterior (AP) view of the pelvis centered over the pubic symphysis and including the proximal third of the femora. Slight internal rotation of the hips allows accurate assessment of the neck–shaft angle. The coccyx should be pointing directly to the symphysis pubis and located about 3 cm above the symphysis pubis if pelvic rotation is neutral.
AP and false-profile views of the involved hip
AP and lateral lumbar spine
Computed tomography, magnetic resonance imaging, and other supplemental studies are indicated if the cause of pain is not evident on plain radiographs.
DIFFERENTIAL DIAGNOSIS
 Lumbar spine pathology
Lumbar spine pathology
Spinal stenosis and neurogenic claudication
Herniated nucleus pulposus
Degenerative disc disease or spondylitis
 Sacroiliac joint pathology
Sacroiliac joint pathology
 Trochanteric bursitis
Trochanteric bursitis
 Tendinopathy of the gluteus medius or minimus
Tendinopathy of the gluteus medius or minimus
 Iliopsoas bursitis
Iliopsoas bursitis
 Inguinal hernia
Inguinal hernia
 Vascular claudication
Vascular claudication
 Femoroacetabular impingement without advanced arthritis
Femoroacetabular impingement without advanced arthritis
NONOPERATIVE MANAGEMENT
 Nonoperative options include weight loss, activity modification, physical therapy, injections, pain management, and the use of walking aids. These interventions do not alter the underlying disease process, but they may substantially diminish pain and disability.
Nonoperative options include weight loss, activity modification, physical therapy, injections, pain management, and the use of walking aids. These interventions do not alter the underlying disease process, but they may substantially diminish pain and disability.
SURGICAL MANAGEMENT
 Cemented THA has been a highly successful operation. Significant early complications are uncommon, and patient outcomes are outstanding in the short and intermediate term. Long-term outcomes beyond 10 to 15 years are limited by component wear, fixation failure, and biologic reaction to wear debris.
Cemented THA has been a highly successful operation. Significant early complications are uncommon, and patient outcomes are outstanding in the short and intermediate term. Long-term outcomes beyond 10 to 15 years are limited by component wear, fixation failure, and biologic reaction to wear debris.
 In most reported series,1,5–8,17 fixation has been the limiting factor for the survival of hip implants in patients with long life expectancies.
In most reported series,1,5–8,17 fixation has been the limiting factor for the survival of hip implants in patients with long life expectancies.
 The durability of cement fixation is highly dependent on meticulous surgical technique. Bone cement is vulnerable to failure under tension and shear, which can be caused by gaps in the cement mantle. Stress risers increase the risk of cement fracture.
The durability of cement fixation is highly dependent on meticulous surgical technique. Bone cement is vulnerable to failure under tension and shear, which can be caused by gaps in the cement mantle. Stress risers increase the risk of cement fracture.
 Improvements in cement technique have resulted in a reduction in the rates of aseptic loosening of femoral components.13,17
Improvements in cement technique have resulted in a reduction in the rates of aseptic loosening of femoral components.13,17
 Acetabular cement fixation remains challenging for many surgeons, with variable results over the long term.3,6,7,9,22 Appearance of the bone–cement interface on the immediate postoperative radiograph predicts the durability of cemented acetabular fixation.22
Acetabular cement fixation remains challenging for many surgeons, with variable results over the long term.3,6,7,9,22 Appearance of the bone–cement interface on the immediate postoperative radiograph predicts the durability of cemented acetabular fixation.22
Surgeons who consistently achieve good cement technique can expect reproducible long-term results.
Preoperative Planning
Indications
 Reproducible, durable, long-term outcomes using cement fixation have been achieved in older, lighter weight patients, particularly women, with low to moderate activity levels and relatively normal anatomy of the pelvis and proximal femur. If THA is indicated for such a patient, cement fixation remains an excellent option for both components.
Reproducible, durable, long-term outcomes using cement fixation have been achieved in older, lighter weight patients, particularly women, with low to moderate activity levels and relatively normal anatomy of the pelvis and proximal femur. If THA is indicated for such a patient, cement fixation remains an excellent option for both components.
 When distorted femoral anatomy interferes with the use of standard press-fit prostheses, cement fixation may be the best and simplest option.
When distorted femoral anatomy interferes with the use of standard press-fit prostheses, cement fixation may be the best and simplest option.
 Cement fixation may also be the best option in pathologic bone associated with tumor or radiation or in any other situation in which bone in- or ongrowth cannot be anticipated.
Cement fixation may also be the best option in pathologic bone associated with tumor or radiation or in any other situation in which bone in- or ongrowth cannot be anticipated.
 Cement fixation of the femoral component increases the quantity of fat and marrow embolization that occurs with THA. We therefore prefer to avoid cement fixation in patients with significant cardiopulmonary disease.
Cement fixation of the femoral component increases the quantity of fat and marrow embolization that occurs with THA. We therefore prefer to avoid cement fixation in patients with significant cardiopulmonary disease.
Implant Selection
 Choice of prosthesis should be based on critical review of the published outcomes and the surgeon’s familiarity with the implant. This includes design features and rationale, instrumentation, and potential technical pitfalls.
Choice of prosthesis should be based on critical review of the published outcomes and the surgeon’s familiarity with the implant. This includes design features and rationale, instrumentation, and potential technical pitfalls.
 It is generally agreed that the optimal cemented acetabular component is all polyethylene (FIG 1), with multiple pegs to ensure concentric insertion within a cement mantle of appropriate thickness and a peripheral flange to optimize pressurization of cement during component insertion. Recent data challenge the value of polyethylene pegs, demonstrating an association with radiographic evidence of loosening.11
It is generally agreed that the optimal cemented acetabular component is all polyethylene (FIG 1), with multiple pegs to ensure concentric insertion within a cement mantle of appropriate thickness and a peripheral flange to optimize pressurization of cement during component insertion. Recent data challenge the value of polyethylene pegs, demonstrating an association with radiographic evidence of loosening.11

 On the femoral side, there has been debate whether the optimal prosthesis is roughened to allow interdigitation of cement and rigid fixation at the cement–prosthesis interface or polished and tapered to allow slight subsidence into a stable position without generating wear particles.
On the femoral side, there has been debate whether the optimal prosthesis is roughened to allow interdigitation of cement and rigid fixation at the cement–prosthesis interface or polished and tapered to allow slight subsidence into a stable position without generating wear particles.
Both design philosophies have resulted in good to excellent long-term results when properly employed.13,17
Conversely, simply roughening the surface of a successful smooth stem has led to a surprising number of early failures of fixation.13
Polished and tapered femoral stems have emerged as the most commonly used cemented stems worldwide.
Templating
 Once the implant system has been chosen, templates can be compared to patient radiographs to predict implant size and determine the implant placement that will best reconstruct the patient’s center of rotation, offset, and leg length.
Once the implant system has been chosen, templates can be compared to patient radiographs to predict implant size and determine the implant placement that will best reconstruct the patient’s center of rotation, offset, and leg length.
 A horizontal reference line is drawn between the inferior tips of the acetabular teardrops (FIG 2A). Both lesser trochanters are marked at their medial points (the medial tip of the lesser trochanter is the most reproducible landmark on the proximal femur radiographically). The perpendicular distance between the interteardrop line and the medial point of the lesser trochanter is measured for each hip (represented by the solid vertical lines in FIG 2A).
A horizontal reference line is drawn between the inferior tips of the acetabular teardrops (FIG 2A). Both lesser trochanters are marked at their medial points (the medial tip of the lesser trochanter is the most reproducible landmark on the proximal femur radiographically). The perpendicular distance between the interteardrop line and the medial point of the lesser trochanter is measured for each hip (represented by the solid vertical lines in FIG 2A).
The LLD is calculated by subtracting the value measured for the nonoperative hip from the value obtained for the operative hip.
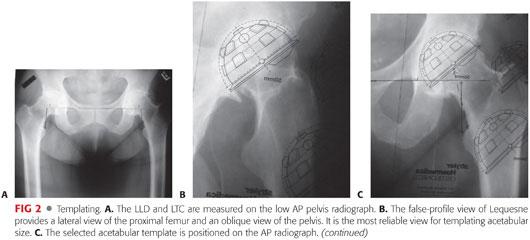
 The centers of the femoral heads are marked. These are the preoperative centers of rotation of the hip joints. A mark is also placed on the superior aspect of the lesser trochanter on the operative hip (this can be identified intraoperatively). The distance between the superior aspect of the lesser trochanter and the center of the femoral head (LTC) (represented by the dashed lines in FIG 2A) is measured and recorded for each hip.
The centers of the femoral heads are marked. These are the preoperative centers of rotation of the hip joints. A mark is also placed on the superior aspect of the lesser trochanter on the operative hip (this can be identified intraoperatively). The distance between the superior aspect of the lesser trochanter and the center of the femoral head (LTC) (represented by the dashed lines in FIG 2A) is measured and recorded for each hip.
 Selection of the appropriate acetabular component requires a measurement of the acetabular size. Cemented socket templating accounts for a 2-mm cement mantle in approximating the reamed hemispherical cavity. Implant size is estimated most accurately on the false-profile radiograph (FIG 2B).
Selection of the appropriate acetabular component requires a measurement of the acetabular size. Cemented socket templating accounts for a 2-mm cement mantle in approximating the reamed hemispherical cavity. Implant size is estimated most accurately on the false-profile radiograph (FIG 2B).
 The acetabular template is positioned on the AP radiograph in 40 to 45 degrees of abduction,18,25 with its inferomedial border placed approximately 10 mm lateral to the teardrop (FIG 2C). The prosthesis should remain at or lateral to the medial floor of the acetabulum, and the superolateral corner of the component should fall near the superolateral border of the acetabulum.
The acetabular template is positioned on the AP radiograph in 40 to 45 degrees of abduction,18,25 with its inferomedial border placed approximately 10 mm lateral to the teardrop (FIG 2C). The prosthesis should remain at or lateral to the medial floor of the acetabulum, and the superolateral corner of the component should fall near the superolateral border of the acetabulum.
Incomplete coverage of the acetabular component (up to 20%) may be acceptable.
 Once the desired position of the acetabular component is selected, the new center of rotation of the hip is determined using the template. In most cases, the goal is to recreate the normal anatomic center of rotation. Changing the center of rotation up to 10 mm may be acceptable to optimize bone stock for component fixation. Such a change may be necessary in cases of dysplasia with a high hip center.
Once the desired position of the acetabular component is selected, the new center of rotation of the hip is determined using the template. In most cases, the goal is to recreate the normal anatomic center of rotation. Changing the center of rotation up to 10 mm may be acceptable to optimize bone stock for component fixation. Such a change may be necessary in cases of dysplasia with a high hip center.
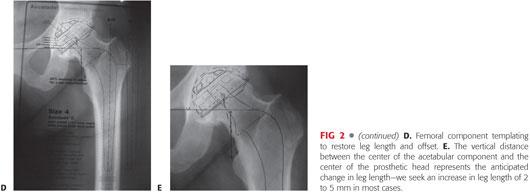
 Attention then is turned to templating the femoral component. The goal is to choose an implant that permits an adequate cement mantle without excessive removal of cancellous bone and restores anatomic leg length and offset.
Attention then is turned to templating the femoral component. The goal is to choose an implant that permits an adequate cement mantle without excessive removal of cancellous bone and restores anatomic leg length and offset.
 The template is placed in neutral position in the femoral canal. Its proximal–distal position should be selected on the basis of the bony constraints, the desire for a circumferential 2-mm cement mantle, and the goal of restoring leg length (FIG 2D).
The template is placed in neutral position in the femoral canal. Its proximal–distal position should be selected on the basis of the bony constraints, the desire for a circumferential 2-mm cement mantle, and the goal of restoring leg length (FIG 2D).
Most implant systems are available with standard or enhanced offset necks; the implant that optimizes the patient’s offset in relation to the new center of rotation of the socket is chosen.
Modular femoral heads with the option of plus and minus sizes allow the surgeon to lengthen or shorten the femoral neck, affecting both leg length and offset.
 Once the optimal component position is selected, the level of the femoral neck osteotomy is marked, and the distance from the lesser trochanter is recorded.
Once the optimal component position is selected, the level of the femoral neck osteotomy is marked, and the distance from the lesser trochanter is recorded.
 After marking socket and femoral component positions on the radiograph, the vertical distance between the center of the acetabular component and the center of the femoral head will approximate the leg length correction (FIG 2E).
After marking socket and femoral component positions on the radiograph, the vertical distance between the center of the acetabular component and the center of the femoral head will approximate the leg length correction (FIG 2E).
The goals of leg length equality and optimal stability should be balanced. In most cases, optimal stability can be achieved with 0 to 5 mm of lengthening compared to the prearthritic state.23
Positioning
 Positioning of the patient depends on the choice of surgical exposure.
Positioning of the patient depends on the choice of surgical exposure.
 For the posterolateral approach to the hip, we use the lateral decubitus position with the pelvis secured to prevent rotation.
For the posterolateral approach to the hip, we use the lateral decubitus position with the pelvis secured to prevent rotation.
An axillary roll is used to prevent injury to the brachial plexus, and all bony prominences are carefully padded to avoid pressure-related complications.
Many surgeons attempt to establish a fixed relationship between the pelvis and the floor to allow positioning of the acetabular component in reference to the plane of the floor. The use of internal landmarks is more reproducible and permits the surgeon more freedom in positioning.
We tilt the table toward the surgeon during acetabular preparation and component insertion, optimizing visualization. A backrest is used to stabilize the patient during this maneuver.
 For the direct anterior approach to the hip, we place the patient in the supine position on a radiolucent table with a table attachment used to facilitate femoral elevation. Custom traction tables and table attachments are also available but increase reliance on nonsterile personnel.
For the direct anterior approach to the hip, we place the patient in the supine position on a radiolucent table with a table attachment used to facilitate femoral elevation. Custom traction tables and table attachments are also available but increase reliance on nonsterile personnel.
Approach
 Multiple surgical approaches can adequately expose the hip joint for THA.
Multiple surgical approaches can adequately expose the hip joint for THA.
 The posterolateral approach is desirable for its excellent extensile exposure and avoidance of trauma to the abductor mechanism. With modern techniques of posterior soft tissue repair and implant positioning that restore the center of rotation, offset, leg length, and combined anteversion,2 dislocation rates are comparable to those observed with other approaches.21
The posterolateral approach is desirable for its excellent extensile exposure and avoidance of trauma to the abductor mechanism. With modern techniques of posterior soft tissue repair and implant positioning that restore the center of rotation, offset, leg length, and combined anteversion,2 dislocation rates are comparable to those observed with other approaches.21
 The direct anterior approach is gaining popularity for its preservation of the gluteus maximus and minimal disruption of the short external rotators as well as improved ability to use intraoperative fluoroscopy and directly assess LLD. There is an associated rate of lateral femoral cutaneous nerve injury. We have used this approach successfully for cementless and cemented femoral applications but have not routinely cemented the acetabulum with this approach.
The direct anterior approach is gaining popularity for its preservation of the gluteus maximus and minimal disruption of the short external rotators as well as improved ability to use intraoperative fluoroscopy and directly assess LLD. There is an associated rate of lateral femoral cutaneous nerve injury. We have used this approach successfully for cementless and cemented femoral applications but have not routinely cemented the acetabulum with this approach.
 Anterolateral, direct lateral Hardinge, and transtrochanteric approaches have all been used with success for the performance of THA. As each of these approaches can compromise the abductor attachment, they have not been used routinely in our practice.
Anterolateral, direct lateral Hardinge, and transtrochanteric approaches have all been used with success for the performance of THA. As each of these approaches can compromise the abductor attachment, they have not been used routinely in our practice.
TECHNIQUES
 Exposure (Posterior Approach)
Exposure (Posterior Approach)
A gently curved skin incision is made starting posterior and slightly proximal to the tip of the greater trochanter, passing about 1 cm posterior to the most prominent point of the greater trochanter on the lateral aspect of the femur and distally along the shaft of the femur to approximately the level of the gluteus maximus insertion.
The iliotibial band is incised slightly anterior to the line of the skin incision so that the fascial incision extends distally from the most prominent point of the trochanter and remains 5 to 10 mm anterior to the insertion of the gluteus maximus tendon into the proximal femur.
The proximal portion of the fascial incision is performed in line with the fibers of the underlying gluteus maximus. The muscle fibers of the gluteus maximus are split bluntly.
Partial or complete release of the gluteus maximus insertion into the linea aspera can be performed at this time. This is seldom necessary for exposure but may reduce the small risk of postoperative sciatic nerve palsy.15
The hip is internally rotated and release of the quadratus femoris off the posterior femur is performed with the electrocautery. This step is complete when the lesser trochanter is exposed.
The first perforator off the profunda femoris artery is often encountered during this step. This vessel is easily cauterized before it is transected, but hemostasis can be more difficult if it is transected before it is recognized.
Leaving a small cuff of quadratus and attached to the femur laterally facilitates repair, but medial dissection is best carried out close to bone so as to minimize bleeding.
The gluteus medius is retracted anteriorly and proximally so the superior border of the piriformis tendon is clearly visualized.
The piriformis tendon, the conjoint tendon of the obturator internus and gemelli, the obturator externus tendon, and the posterior capsule are released as a single flap from the greater trochanter and the lateral portion of the femoral neck.
Superior and inferior capsulotomies create a quadrangular flap of capsule, tendon, and muscle for repair at the end of the case.
The superior capsulotomy is performed along the inferior edge of the gluteus minimus.
The inferior capsulotomy is performed at approximately the 7:30 position for a right hip, separating the posterior capsule from the inferior capsule. The obturator externus muscle is adjacent to the capsule but is not visible until the lateral portion of the inferior capsulotomy is performed. After initiating the inferior capsulotomy, a retractor placed between the capsule and the underlying obturator externus will protect the muscle and minimize bleeding associated with the capsulotomy.
 Intraoperative Assessment of Leg Length
Intraoperative Assessment of Leg Length
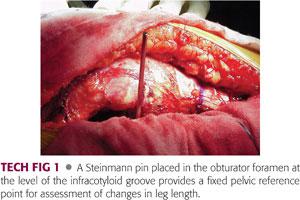
Prior to dislocation of the hip, a Steinmann pin is placed into the obturator foramen at the level of the infracotyloid groove.23 This landmark can be reproducibly identified by passing the pin just distal to the ischium at the level of the acetabulum.
The surgeon should experience a pop as the pin pierces the obturator membrane; the pin should be inserted no further.
The femur is placed in a neutral and reproducible position on the operating table and the position of the vertical Steinmann pin is marked on the femur using the electrocautery and a marking pen (TECH FIG 1).
The Steinmann pin can be replaced later in the case and the mark on the femur provides a reference for assessment of change in leg length.
 Dislocation of Hip and Osteotomy of Femoral Neck
Dislocation of Hip and Osteotomy of Femoral Neck
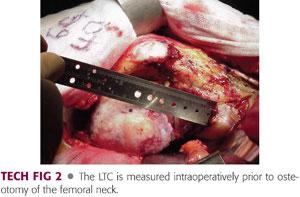
The hip is dislocated posteriorly using gentle flexion, adduction, and internal rotation.
The center of the femoral head is then estimated and marked, and the LTC is measured and recorded (TECH FIG 2).
Reconstruction of the anatomic geometry of the hip, including leg length and offset, is aided by approximate reproduction of this distance.
A slight (<5 mm) increase in the LTC can optimize hip stability without excessive lengthening of the leg or stretching the iliotibial band.
The femoral neck osteotomy is performed at the templated level, aiming at the junction of the femoral neck with the greater trochanter.
The femoral neck can be left a few millimeters longer than templated, allowing for measurement error. Additional bone can easily be removed during femoral preparation using the sagittal saw or the calcar planar.
 Acetabular Exposure
Acetabular Exposure
Wide exposure of the acetabulum is achieved by translating the femur anteriorly.
This typically requires release of the superior capsule, which should be divided at its acetabular insertion with care to avoid trauma to the overlying gluteus minimus.
In stiff hips, further mobility can be achieved by releasing the reflected head of the rectus femoris muscle, which becomes evident after the anterior portion of the superior capsular release is performed.
Release of the tendinous insertion of the gluteus maximus into the linea aspera allows further anterior translation, if necessary.
The labrum should be resected, but the transverse acetabular ligament should be preserved to provide a landmark for the placement of the inferior portion of the acetabular component and a restraint to the extrusion of cement inferiorly during cement pressurization and component insertion.
The pulvinar should be removed from the fovea using electrocautery to allow visualization of the medial wall of the acetabulum.
 Acetabular Preparation
Acetabular Preparation
A slightly undersized reamer is used initially to ensure appropriate medialization without penetrating the medial wall, followed by sequential concentric reaming until the blush of cancellous bone is seen in the pubis anteriorly and the ischium posteriorly.
Historically, the first reamer chosen was several sizes smaller than the templated size.
Starting with a reamer the size of the removed femoral head saves time and minimizes eccentric reaming.
Most of the strong subchondral bone of the ilium in the superior aspect of the acetabulum is typically preserved to provide support for the prosthesis. However, sclerotic bone must be penetrated sufficiently to permit cement interdigitation using multiple holes with a high-speed burr.
A randomized controlled clinical trial demonstrated the significantly improved radiographic appearance of the cement mantle with careful removal of most of the subchondral bone to allow cement interdigitation into cancellous bone of the roof of the acetabulum.12
The appropriate position for the acetabular component is selected using a trial prosthesis. Insertion of the trial component should be easy and free of bone or soft tissue obstruction to allow for unencumbered insertion of the actual component. If the margins of the acetabular cavity remain tight, it can be reamed an additional 1 mm at the periphery.
Internal landmarks used for positioning the acetabular cup include the anterior wall and pubic ramus, posterior wall, transverse acetabular ligament, and superior acetabular rim.
With normal acetabular morphology, positioning the prosthesis just within the confines of the acetabulum with a small amount of implant exposed posterosuperiorly and the inferior aspect tucked inside and parallel to the transverse acetabular ligament ensures appropriate component abduction of 40 to 45 degrees and anteversion of 10 to 20 degrees.
In cases with large anterior osteophytes or preoperative acetabular retroversion as noted by a positive crossover sign, the posterior wall and the transverse acetabular ligament are used preferentially to gauge proper anteversion. Anterior osteophytes should be debulked using a burr or an osteotome; this reduces the risk of anterior bony impingement with hip flexion and internal rotation.
Once the appropriate component position is selected using the trial, it can be marked on the bone using methylene blue, and the relationship of the component to the aforementioned landmarks can be noted visually to assist in placement of the final component (TECH FIG 3A).
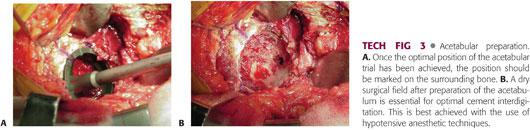
A high-speed burr is then used to create holes in the pubis, ischium, and ilium for cement intrusion and “macrolock” to complement the “microlock” achieved by interdigitation in bony trabeculae of cancellous bone.
If acetabular cysts are present, these are débrided and the sclerotic margins are removed using the burr.
A dry operative field free of debris is necessary for maximal cement interdigitation into cancellous bone (TECH FIG 3B).
This is achieved by the use of hypotensive regional anesthesia with arterial pressure in the range of 45 to 70 mm Hg and pulse irrigation to remove fat and blood followed by drying with a sponge, with or without local use of epinephrine.
Although it is not our practice, others have demonstrated improved cement intrusion when suction aspiration of the ilium was performed at the time of cementing to help maintain a dry bone surface.14
 Cementing the Acetabular Component
Cementing the Acetabular Component
Cement should be doughy but still relatively low in viscosity when it is placed in the acetabulum. Uniform simultaneous cement pressurization is then achieved using a rubber balloon that is pressed into the acetabulum (TECH FIG 4A).
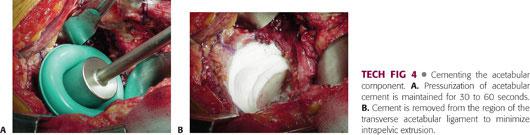
After pressurization has been maintained for 30 to 60 seconds, the balloon is removed, and the transverse acetabular ligament is cleared of cement (TECH FIG 4B). This minimizes intrapelvic extrusion and allows visualization of the floor of the acetabulum to guide placement of the acetabular component.
The acetabular component is then inserted, with care to match the abduction and anteversion selected at the time the trial prosthesis was inserted. The component should have an outer diameter 2 mm smaller than that of the final reamer, allowing for an adequate cement mantle.
Extra cement is removed while pressure is maintained on the acetabular component using a Charnley pusher centrally to minimize angular forces on the cement mantle until the cement has hardened.
 Femoral Preparation
Femoral Preparation
Exposure requires proper delivery of the proximal femur out of the wound by flexion, adduction, and internal rotation. Difficulty achieving this position can be remedied by release of the gluteus maximus tendon.
The starting point for entry into the femoral canal is in the posterolateral femoral neck. This allows cylindrical reamers and straight broaches to be inserted along the anatomic axis of the proximal femoral diaphysis while maintaining a uniform cement mantle despite the proximal femoral bow.
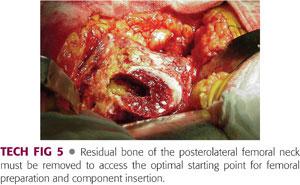
To achieve the appropriate starting point, all residual soft tissue must be removed from the posterolateral femoral neck and remaining bone must be removed using a high-speed burr or other tool.
Many surgeons successfully use a box osteotome to achieve this goal, although it does not have the precision of the burr (TECH FIG 5).
Once the starting point has been prepared, a conical canal-finding reamer is introduced to aid in the identification of the anatomic axis of the femur. The entry point into the femur is opened, while reaming of the diaphyseal endosteum is minimized. Broach preparation of the canal without extensive reaming preserves cancellous bone to permit optimal cement interdigitation.
Sequential broaching is then performed, with care to insert the broaches in appropriate anteversion (typically 10 to 15 degrees). This is achieved by following the patient’s native version, unless the patient has significant deformity of the proximal femur or the acetabular component is known to be in excessive anteversion or retroversion.
The degree of anteversion is best assessed visually if the assistant holds the tibia perpendicular to the plane of the floor.
Sequential broaching is continued until torsional stability is achieved at a depth of broach insertion that brings the proximal surface of the broach into the plane of the neck cut.
If careful preoperative templating was performed, this should result in restoration of leg length and offset with the implant system being used. This can be confirmed following the attachment of trial necks and heads.
Many hip systems have options for standard or extended offset necks; these can be defined by the amount of offset or by the neck–shaft angle.
In general, the neck that best recreated the anatomic geometry on preoperative templating should be selected.
However, be aware that radiographs may underestimate offset if the hip is not internally rotated to bring the femoral neck perpendicular to the x-ray beam.
A trial femoral head is selected using the preoperative plan and attempting to recreate or minimally increase the LTC.
 Assessment of the Reconstruction and Soft Tissue Balance Using Trial Components
Assessment of the Reconstruction and Soft Tissue Balance Using Trial Components
A trial reduction is performed and the adequacy of the reconstruction is assessed using four principal maneuvers:
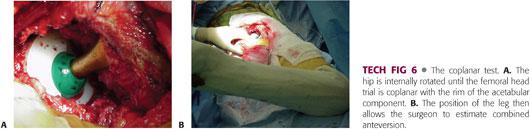
First, the hip is internally rotated until the femoral head trial and acetabular component are coplanar (TECH FIG 6A,B) and the knee is bent 90 degrees.
• If the coronal plane of the pelvis is perpendicular to the floor, the angle between the tibia and the floor is the combined anteversion of the femoral and acetabular components.16
• Combined anteversion of 35 to 40 degrees is optimal in women, whereas somewhat less anteversion is desirable in men, who usually have less lumbar lordosis.
Second, the hip is externally rotated with the hip and knee in extension. The anterior capsule should be loose enough to allow external rotation of the femur so that the greater trochanter approaches one fingerbreadth away from the ischium but not so loose as to allow impingement of the trochanter against the ischium or of the prosthetic neck against the posterior socket.
Third, the Steinmann pin is replaced in the obturator foramen at the level of the infracotyloid groove and the relative lengthening or shortening of the leg is measured and noted.
• In general, the goal is to increase the leg length sufficiently to eliminate any preoperative LLD. An additional 2 or 3 mm of lengthening can optimize the perceived stability of the hip, but additional lengthening beyond 5 mm can generate a clinically meaningful LLD. This may vary with preoperative clinical LLD and other factors.
Fourth, the hip is flexed and internally rotated and stability is assessed. The surgeon should feel a clear soft tissue resistance prior to dislocation rather than a smooth unimpeded motion.
Additional information may be gained from the Ober test, in which the knee is flexed 90 degrees and the hip is extended to neutral and abducted. The knee is then released while the examiner continues to support the foot.
If the offset has been substantially increased, the knee will remain elevated (ie, the hip will remain abducted), indicating tightness of the iliotibial band.
Results of this test are meaningless unless they are compared to preoperative findings, as the iliotibial band may be tight preoperatively.
A commonly used test that provides more limited information is the shuck or push–pull test, in which an assistant applies traction on the femur with the hip reduced but internally rotated and the surgeon subjectively assesses the extent to which the femoral head can be distracted from the acetabulum.
There should be some give with push–pull, but the assistant should be unable to completely dislocate the hip with simple traction.
Used in isolation, this test may lead the surgeon to overlengthen the leg.
If the hip is found to be too loose, several options exist:
The size of the femoral stem can be increased. Larger stems may also have longer necks, depending on the implant system.
If leg length is appropriate but offset is insufficient, the surgeon can switch from a standard to an extended-offset stem.
A plus-sized modular head can be used. We recommend against use of skirted heads, but modern implant systems typically provide femoral heads of several lengths without the need for a skirt. We recommend against using the longest femoral head without a skirt. If the final reconstruction varies from the trial reconstruction, the surgeon is left without the option of further increasing leg length and offset.
If the anterior capsule is found to be tight in a hip with an otherwise acceptable reconstruction, we advocate anterior capsulotomy to balance the hip.
If the hip is too tight, with excessive anterior capsular tightness, a positive Ober test, and excessive lengthening, several options exist:
The femoral trial can be downsized or implanted deeper into the femur.
A minus-sized femoral head can be selected. We recommend against using the shortest femoral head. If the final reconstruction varies from the trial reconstruction, the surgeon is left without the option of further decreasing leg length and offset.
 Cementing the Femoral Component
Cementing the Femoral Component
The femoral trials are removed and the femur is prepared for cement fixation. A distal cement restrictor (TECH FIG 7) is placed approximately 1 cm past the anticipated depth of stem insertion.
This helps avoid unnecessarily long cement mantles that are difficult to remove at revision and enhances cement pressurization.
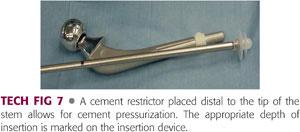
The femoral canal is irrigated using pulse lavage, dried using suction, and packed with vaginal packing or a surgical sponge.
Cement for the femoral side should be prepared under vacuum or using centrifugation, both of which increase cement strength by reducing cement porosity. Cement is then poured into a cement gun. The cement is ready to be injected when it has reached an intermediate viscosity low enough to be inserted with the cement gun and to easily interdigitate in cancellous bone but high enough to allow pressurization.
After ensuring that there is no air in the tip of the cement gun, cement is injected in a retrograde fashion from distal to proximal, allowing the cement to push the cement gun out of the canal.
Once the canal is filled to the level of the neck cut, the tip is removed from the cement gun and replaced with a cement-pressurizing device that occludes the proximal femoral canal.
Any holes in the femoral shaft should be occluded prior to cement pressurization.
As pressurization is performed, cement, fat, and marrow contents should be seen extruding from small vascular foramina in the femoral neck. When the pressurizer is removed from the femur, the void should be filled with more cement.
The surface of the cement is dried with a sponge, and cement is used to coat the femoral stem, concentrating on the metaphyseal region. These measures diminish the amount of blood, fluid, and other debris present in the cement and at the cement–prosthesis interface. Such impurities have been shown to have significant effects on cement strength.
If the femur has a relatively wide diaphysis, addition of a distal centralizer is advised to reduce the risk of varus malpositioning of the stem.
The stem is best inserted when the cement is in the medium dough phase. The amount of time required for the cement to reach this state varies with room temperature and rate of mixing.
Preheating the stem will further reduce cement porosity and accelerate cement polymerization.18
To avoid the creation of voids in the cement mantle, the stem should be inserted in one continuous smooth motion, without adjusting varus/valgus or rotational alignment. Insertion is started by hand, impacting the insertion device with a mallet as needed.
Once the position of the trial stem has been reproduced, gentle pressure is maintained on the stem while excess cement is removed and cement around the stem is pressurized by finger.
When the cement has polymerized, the previously selected trial head is placed on the stem, and the LTC, leg length and soft tissue balance, and combined anteversion are reassessed.
Once the appropriate head is selected, the trunnion of the stem is carefully cleaned and dried, and the implant is gently impacted in place.
The acetabulum is cleared of debris using irrigation and suction, and reduction is performed.
 Soft Tissue Repair and Wound Closure
Soft Tissue Repair and Wound Closure
Injection of the deep soft tissues (ie, hip capsule, gluteus medius tendon, vastus lateralis, and iliotibial band) with a combination of local anesthetic, narcotic, and either corticosteroid or nonsteroidal anti-inflammatory medication results in decreased postoperative pain and narcotic requirements.19
After copious irrigation of all exposed tissues, an extended posterior soft tissue repair is performed (TECH FIG 8).
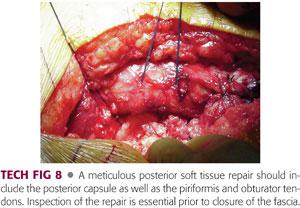
The quadratus femoris is repaired to its insertion, along with repair of the gluteus maximus insertion if this tendon was released.
A figure-8 suture is placed approximating the superior aspect of the piriformis to the superior capsule and/or gluteus minimus tendon; this suture is not tied initially.
Transosseous repair of the short external rotators and posterior capsule to the posteromedial aspect of the greater trochanter is performed.
A nonabsorbable suture is passed through the superolateral portion of the posterior capsular flap and the piriformis tendon in a single pass, with a second pass through the capsule and the conjoint tendon.
A second nonabsorbable suture is passed through the inferolateral portion of the capsular flap and the obturator externus tendon and then again through the capsule.
The two sutures are passed through drill holes in the greater trochanter and tied to each other. Prior to tying the sutures, the leg is abducted and externally rotated, minimizing tension on the posterior soft tissue flap.
The suture connecting the piriformis to the superior capsule and/or abductors is tied last.
The repair should be inspected carefully to make sure that the posterior flap is in intimate contact with the femur before the fascia is closed.
An inadequate repair can easily be revised. If the soft tissues cannot reach the bone, a significant increase in length and/or offset is implied. If this was not planned, femoral modularity can be used to shorten the limb and avoid severe LLD.
The wound is once again copiously irrigated and routine closure of the fascia, subcutaneous tissue, and skin is performed.
PEARLS AND PITFALLS | |
Leg length and offset |
|
Indications |
|
| |
Exposure |
|
Hypotensive anesthesia |
|
POSTOPERATIVE CARE
 Blood management
Blood management
Preoperative medical management of anemia is the best approach to minimizing transfusions.
Preoperative autologous blood donation is not routinely required but can be used to reduce postoperative exposure to allogenic blood in patients with mild preoperative anemia.
Preoperative recombinant human erythropoietin may be considered in patients unable to donate blood.
Allogenic transfusion may be used as indicated for symptomatic anemia related to surgical blood loss.
 Pain control
Pain control
Patient satisfaction is improved by the use of multimodal analgesia protocols,19 combining soft tissue injections at the time of surgery, acetaminophen, nonsteroidal anti-inflammatory medications, and both long- and short-acting narcotics.
These regimens reduce both pain and narcotic requirements, thereby reducing perioperative nausea, emesis, sedation, and confusion and enabling more rapid rehabilitation.
 Intravenous antibiotics
Intravenous antibiotics
Antibiotics are given within 1 hour before surgery and continued postoperatively for 24 hours.
Cefazolin is the preferred antibiotic.
Vancomycin or clindamycin are typically used in the patient allergic to penicillin or cephalosporins. Vancomycin may be preferable, as Staphylococcus epidermidis isolates are often resistant to clindamycin.
 Prophylaxis against venous thromboembolic disease
Prophylaxis against venous thromboembolic disease
Intermittent pneumatic compression devices provide mechanical prophylaxis, which has been proven to reduce the risk of venous thromboembolism (VTE), both as the sole mode of prophylaxis and as an adjunct to pharmacologic prophylaxis.
The optimal pharmacologic prophylaxis remains a matter of debate, but some form of prophylaxis should be started inthe hospital and continued after discharge in the vast majority of patients. We typically use aspirin for patients at low or standard risk of VTE and low or standard risk of surgical site bleeding. Patients with a history of VTE or who are otherwise deemed to be at high risk for thrombosis, as well as those with a prior indication for anticoagulation, are typically kept on extended warfarin prophylaxis after discharge. Patients at high risk of bleeding require an individualized approach. In some such cases, withholding pharmacologic prophylaxis may be appropriate during the period when bleeding is most likely. Mechanical prophylaxis may play a particularly important role in these patients.
Accelerated rehabilitation protocols further reduce the risk of thromboembolic disease and are an important part of most multimodal prophylaxis regimens.
Screening Doppler ultrasounds are no longer recommended.
 Physical therapy
Physical therapy
Posterior hip dislocation precautions are recommended for patients undergoing THA through a posterior approach. Our standard physical therapy regimen is 6 weeks, although precautions can be relaxed earlier in patients who are stiff at their first follow-up visit. Precautions may not be required with other surgical approaches.20 A recent study in which limited posterior precautions (avoiding flexion greater than 100 degrees or marked internal rotation of the hip) were used showed a very low dislocation rate with a modified posterior soft tissue repair.4
Weight bearing is permitted as tolerated with a walker or two crutches starting within 24 hours of surgery.
Patients are weaned off walking aids as tolerated.
 Discharge
Discharge
Most patients can be discharged home 2 to 3 days after surgery.
Patients with other severely affected joints, difficult home environments, or poor social support may require a brief period of inpatient rehabilitation.
OUTCOMES
 Relief of hip pain and restoration of function are remarkable after THA. Thigh pain is rare after cemented THA, whereas it is relatively common after noncemented femoral fixation.
Relief of hip pain and restoration of function are remarkable after THA. Thigh pain is rare after cemented THA, whereas it is relatively common after noncemented femoral fixation.
 The clinical success of cemented THA has been documented at long-term follow-up (FIG 3). Although function may decline with age and comorbidities, 94% of patients followed for 30 years were free from hip pain or reported minimal discomfort.27
The clinical success of cemented THA has been documented at long-term follow-up (FIG 3). Although function may decline with age and comorbidities, 94% of patients followed for 30 years were free from hip pain or reported minimal discomfort.27
 Minimum 25-year follow-up data after cemented THA using first-generation cement techniques are available.1,6,7 Each center reported a single-surgeon series consisting of consecutive cases performed in the late 1960s and early 1970s.
Minimum 25-year follow-up data after cemented THA using first-generation cement techniques are available.1,6,7 Each center reported a single-surgeon series consisting of consecutive cases performed in the late 1960s and early 1970s.
Implant survivorship was 94% at 10 years, 90% at 15 years, 84% to 85% at 20 years, 77% to 81% at 25 years, and 68% at 30 years.
Revision with removal of at least one component was required in 12% of hips at 30-year follow-up,7 with the remainder of the original implants either still functioning well in vivo (7%) or in place at the time of patient death (81%).
 Minimum 20-year follow-up data after cemented THA using improved cement technique in the 1970s and early 1980s are also available.3,5,24,26
Minimum 20-year follow-up data after cemented THA using improved cement technique in the 1970s and early 1980s are also available.3,5,24,26
Revision with removal of at least one component has been required in 3% to 10% of patients at 10 to 15 years and in 5% to 12% of patients at 20 to 25 years.
 Reasons for revision
Reasons for revision
Aseptic loosening accounts for most revision procedures after cemented THA, with rates ranging from 62% to 100%.1,3,17,26
Deep infection, recurrent dislocation, and periprosthetic fracture account for most other revisions.
Less common reasons for revision after cemented THA include osteolysis, isolated polyethylene wear, and technical errors such as LLD.
Component fracture, a major cause of revision with early implant systems, is very uncommon with modern implants.
COMPLICATIONS
 Embolism of fat and bone marrow occurs whenever the marrow space of a long bone is instrumented but seldom results in fat embolism syndrome. Cement fixation of the femoral component may increase the quantity of fat displaced, the consequent pulmonary shunt, and the risk of fat embolism syndrome.24 For this reason, we avoid cement fixation in patients with significant cardiopulmonary disease.
Embolism of fat and bone marrow occurs whenever the marrow space of a long bone is instrumented but seldom results in fat embolism syndrome. Cement fixation of the femoral component may increase the quantity of fat displaced, the consequent pulmonary shunt, and the risk of fat embolism syndrome.24 For this reason, we avoid cement fixation in patients with significant cardiopulmonary disease.
 VTE is common in THA if prophylaxis is not used. Most prophylactic regimens are associated with low rates of symptomatic deep vein thrombosis (DVT) and pulmonary embolism, with fatal pulmonary embolism occurring in fewer than 0.5% of patients. Aggressive pharmacologic anticoagulation has been proven to reduce the rate of asymptomatic DVT, but no regimen has been found to decrease the low rate of fatal pulmonary embolism.
VTE is common in THA if prophylaxis is not used. Most prophylactic regimens are associated with low rates of symptomatic deep vein thrombosis (DVT) and pulmonary embolism, with fatal pulmonary embolism occurring in fewer than 0.5% of patients. Aggressive pharmacologic anticoagulation has been proven to reduce the rate of asymptomatic DVT, but no regimen has been found to decrease the low rate of fatal pulmonary embolism.
 Cardiopulmonary complications are uncommon with appropriate preoperative medical optimization and conservative surgical indications, but at-risk patients should be monitored carefully in the perioperative period.
Cardiopulmonary complications are uncommon with appropriate preoperative medical optimization and conservative surgical indications, but at-risk patients should be monitored carefully in the perioperative period.
 Clinically meaningful LLD is an avoidable complication in most patients. In a prospective study,23 the methods for equalizing leg lengths described in this chapter resulted in postoperative LLD that averaged +2.6 mm (range, −7 to +9 mm), with 87% having inequality of 6 mm or less. None of the patients reported symptoms of LLD or required the use of a shoe lift.
Clinically meaningful LLD is an avoidable complication in most patients. In a prospective study,23 the methods for equalizing leg lengths described in this chapter resulted in postoperative LLD that averaged +2.6 mm (range, −7 to +9 mm), with 87% having inequality of 6 mm or less. None of the patients reported symptoms of LLD or required the use of a shoe lift.
 Infection can be a devastating complication after THA.
Infection can be a devastating complication after THA.
Perioperative intravenous antibiotics and antibiotic-laden bone cement10 have both been associated with decreased risk of deep infection.
Laminar flow and body exhaust suits have been demonstrated to decrease the risk of infection in the setting of inconsistent antibiotic use, but additive benefit in the setting of consistent use of prophylactic antibiotics is unproven.
The use of iodine-impregnated adhesive plastic drapes and the minimization of operating room traffic may also reduce bacteria counts in the surgical wound.
 Dislocation after THA is one of the more common causes of early revision surgery. Dislocation risk is minimized when the reconstruction restores leg length, offset, and center of rotation, with appropriate femoral and acetabular anteversion. Improving the head–neck ratio with the use of large-diameter heads can reduce dislocation risk, but polyethylene thickness should not be compromised in cemented THA, as thin polyethylene implants transfer load to the cement mantle less uniformly.
Dislocation after THA is one of the more common causes of early revision surgery. Dislocation risk is minimized when the reconstruction restores leg length, offset, and center of rotation, with appropriate femoral and acetabular anteversion. Improving the head–neck ratio with the use of large-diameter heads can reduce dislocation risk, but polyethylene thickness should not be compromised in cemented THA, as thin polyethylene implants transfer load to the cement mantle less uniformly.
 Periprosthetic fracture can occur intra- or postoperatively. The key to management is intraoperative recognition, as most fractures can be managed expediently at the time of surgery. If an appropriate starting point is used for femoral preparation, intraoperative fractures in primary cemented THA are uncommon. Postoperative fractures typically are associated with trauma, often in the setting of osteolysis, and their management is beyond the scope of this chapter.
Periprosthetic fracture can occur intra- or postoperatively. The key to management is intraoperative recognition, as most fractures can be managed expediently at the time of surgery. If an appropriate starting point is used for femoral preparation, intraoperative fractures in primary cemented THA are uncommon. Postoperative fractures typically are associated with trauma, often in the setting of osteolysis, and their management is beyond the scope of this chapter.
 Aseptic loosening is the most common cause of failure after cemented THA. The risk of aseptic loosening can be decreased by the use of well-designed implants and modern cement techniques. Nevertheless, several patient factors influence the rates of aseptic loosening after cemented THA.
Aseptic loosening is the most common cause of failure after cemented THA. The risk of aseptic loosening can be decreased by the use of well-designed implants and modern cement techniques. Nevertheless, several patient factors influence the rates of aseptic loosening after cemented THA.
Male gender is strongly associated with increased risk of revision for aseptic loosening.1
The severity of acetabular dysplasia is also a risk factor for aseptic loosening, with increased rates of revision associated with Crowe types III and IV hip dysplasia compared with those with less or no dysplasia.8
Inflammatory arthritis is associated with decreased risk of revision for aseptic loosening.1
Patient age at time of surgery is inversely correlated with risk of revision for aseptic loosening. Twenty-five-year survivorship free of revision for aseptic loosening was 68.7% in patients who were younger than 40 years of age at the time of primary arthroplasty and 100% in patients older than 80 years of age, with incremental increases in survival observed for each decade of increased age between 40 and 80 years.1
 Osteolysis, a common cause of failure in uncemented implants, is less common after cemented THA and possibly related to decreased polyethylene wear in cemented THA. Although ballooning osteolysis is uncommon when cement is used, fixation failure in cemented THA is related to the biologic reaction to wear debris.
Osteolysis, a common cause of failure in uncemented implants, is less common after cemented THA and possibly related to decreased polyethylene wear in cemented THA. Although ballooning osteolysis is uncommon when cement is used, fixation failure in cemented THA is related to the biologic reaction to wear debris.
 Sciatic nerve palsy is an uncommon complication after cemented THA. It most commonly occurs when the operated extremity is lengthened substantially after a long-standing (especially congenital) shortening of the limb, resulting in traction-related nerve ischemia. We routinely palpate the sciatic nerve before the hip is dislocated and again after the arthroplasty is performed to assess whether the tension in the nerve has been excessively increased.
Sciatic nerve palsy is an uncommon complication after cemented THA. It most commonly occurs when the operated extremity is lengthened substantially after a long-standing (especially congenital) shortening of the limb, resulting in traction-related nerve ischemia. We routinely palpate the sciatic nerve before the hip is dislocated and again after the arthroplasty is performed to assess whether the tension in the nerve has been excessively increased.
The sciatic nerve may also be compressed under the tendon of the gluteus maximus during surgery if the hip is maintained in severe flexion and internal rotation. For this reason, Hurd et al15 recommended routine release of the gluteus maximus tendon during THA.
REFERENCES
1. Berry DJ, Harmsen WS, Cabanela ME, et al. Twenty-five-year survivorship of two thousand consecutive primary Charnley total hip replacements: factors affecting survivorship of acetabular and femoral components. J Bone Joint Surg Am 2002;84:171–177.
2. Biedermann R, Tonin A, Krismer M, et al. Reducing the risk of dislocation after total hip arthroplasty: the effect of orientation of the acetabular component. J Bone Joint Surg Br 2005;87:762–769.
3. Bourne RB, Rorabeck CH, Skutek M, et al. The Harris design-2 total hip replacement fixed with so-called second-generation cementing techniques: a ten to fifteen-year follow-up. J Bone Joint Surg Am 1998;80:1775–1780.
4. Brown JA, Pagnano MW. Surgical technique: a simple soft-tissue-only repair of the capsule and external rotators in posterior-approach THA. Clin Orthop Relat Res 2012;470:511–515.
5. Buckwalter AE, Callaghan JJ, Liu SS, et al. Results of Charnley total hip arthroplasty with use of improved femoral cementing techniques: a concise follow-up, at a minimum of twenty-five years, of a previous report. J Bone Joint Surg Am 2006;88:1481–1485.
6. Callaghan JJ, Albright JC, Goetz DD, et al. Charnley total hip arthroplasty with cement: minimum twenty-five-year follow-up. J Bone Joint Surg Am 2000;82:487–497.
7. Callaghan JJ, Templeton JE, Liu SS, et al. Results of Charnley total hip arthroplasty at a minimum of thirty years: a concise follow-up of a previous report. J Bone Joint Surg Am 2004;86:690–695.
8. Chougle A, Hemmady MV, Hodgkinson JP. Severity of hip dysplasia and loosening of the socket in cemented total hip replacement: a long-term follow-up. J Bone Joint Surg Br 2005;87:16–20.
9. Crites BM, Berend ME, Ritter MA. Technical considerations of cemented acetabular components: a 30-year evaluation. Clin Orthop Relat Res 2000;381:114–119.
10. Engesaeter LB, Lie SA, Espehaug B, et al. Antibiotic prophylaxis in total hip arthroplasty: effects of antibiotic prophylaxis systemically and in bone cement on the revision rate of 22,170 primary hip replacements followed 0–14 years in the Norwegian Arthroplasty Register. Acta Orthop Scand 2003;74:644–651.
11. Faris PM, Ritter MA, Keating EM, et al. The cemented all-polyethylene acetabular cup: factors affecting survival with emphasis on the integrated polyethylene spacer: an analysis of the effect of cement spacers, cement mantle thickness, and acetabular angle on the survival of total hip arthroplasty. J Arthroplasty 2006;21:191–198.
12. Flivik G, Kristiansson I, Kesteris U, et al. Is removal of subchondral bone plate advantageous in cemented cup fixation? A randomized RSA study. Clin Orthop Relat Res 2006;448:164–172.
13. Herberts P, Malchau H. How outcome studies have changed total hip arthroplasty practices in Sweden. Clin Orthop Relat Res 1997;344:44–60.
14. Hogan N, Azhar A, Brady O. An improved acetabular cementing technique in total hip arthroplasty: aspiration of the iliac wing. J Bone Joint Surg Br 2005;87:1216–1219.
15. Hurd JL, Potter HG, Dua V, et al. Sciatic nerve palsy after primary total hip arthroplasty: a new perspective. J Arthroplasty 2006;21:796–802.
16. Lucas DH, Scott RB. Coplanar test: the Ranawat sig. A specific maneuver to assess component position in total hip arthroplasty. J Orthop Tech 1994;2:59.
17. Malchau H, Herberts P, Eisler T, et al. The Swedish Total Hip Replacement Register. J Bone Joint Surg Am 2002;84(suppl 2):2–20.
18. Parks ML, Walsh HA, Salvati EA, et al. Effect of increasing temperature on the properties of four bone cements. Clin Orthop Relat Res 1998;355:238–248.
19. Parvataneni HK, Shah VP, Howard H, et al. Controlling pain after total hip and knee arthroplasty using a multimodal protocol with local periarticular injections: a prospective, randomized study. J Arthroplasty 2007;22(6):33–38.
20. Peak EL, Parvizi J, Ciminiello M, et al. The role of patient restrictions in reducing the prevalence of early dislocation following total hip arthroplasty. A randomized, prospective study. J Bone Joint Surg Am 2005;87:247–253.
21. Pellicci PM, Bostrom M, Poss R. Posterior approach to total hip replacement using enhanced posterior soft tissue repair. Clin Orthop Relat Res 1998;355:224–228.
22. Ranawat CS, Deshmukh RG, Peters LE, et al. Prediction of the long-term durability of all-polyethylene cemented sockets. Clin Orthop Relat Res 1995;317:89–105.
23. Ranawat CS, Rao RR, Rodriguez JA, et al. Correction of limb-length inequality during total hip arthroplasty. J Arthroplasty 2001;16: 715–720.
24. Ries MD, Lynch F, Rauscher LA, et al. Pulmonary function during and after total hip replacement: findings in patients who have insertion of a femoral component with and without cement. J Bone Joint Surg Am 1993;75:581–587.
25. Siebenrock KA, Leunig M, Ganz R. Periacetabular osteotomy: the Bernese experience. J Bone Joint Surg Am 2001;83:449.
26. Skutek M, Bourne RB, Rorabeck CH, et al. The twenty to twenty-five-year outcomes of the Harris design-2 matte-finished cemented total hip replacement: a concise follow-up of a previous report. J Bone Joint Surg Am 2007;89:814–818.
27. Wroblewski BM, Fleming PA, Siney PD. Charnley low-frictional torque arthroplasty of the hip: 20-to-30 year results. J Bone Joint Surg Br 1999;81:427–430.
< div class='tao-gold-member'>








