Generic name
Trade name
Dose (mcg)
Delivery
FDA-approved indications
Beclomethasone dipropionate monohydrate
Beconase AQ
42
Aqueous spray
1. Seasonal and allergic rhinitis in adults
2. CRSwNP in adults
Beclomethasone dipropionate
Qnasl
80
Nonaqueous spray
Seasonal and perennial allergic rhinitis in adults and adolescents >12 year/o
Budesonide
Rhinocort AQ
32
Aqueous spray
Seasonal and perennial allergic rhinitis in patients >6 year/o
Ciclesonide
Omnaris
50
Aqueous spray
1. Seasonal allergic rhinitis in adults and children >6 year/o
2. Perennial allergic rhinitis in adults and children >12 year/o
Ciclesonide
Zetonna
37
Nonaqueous spray
Seasonal and perennial allergic rhinitis in adults and adolescents >12 year/o
Flunisolide
Nasalide
58
Aqueous spray
Seasonal and perennial allergic rhinitis
Fluticasone propionate
Flonase
50
Aqueous spray
1. Seasonal and perennial allergic rhinitis in patients >4 year/o
2. Non-allergic rhinitis in patients >4 year/o
Fluticasone furoate
Veramyst
27.5
Aqueous spray
Seasonal and perennial allergic rhinitis in patients >2 year/o
Mometasone furoate monohydrate
Nasonex
50
Aqueous spray
1. Seasonal and perennial allergic rhinitis in patients >2 year/o
2. Prophylaxis of seasonal allergic rhinitis in patients >12 year/o
3. CRSwNP in patients >18 year/o
Triamcinolone acetonide
Nasacort AQ
55
Aqueous spray
Seasonal and perennial allergic rhinitis in patients >2 year/o
The ability to treat inflammation within the paranasal sinuses depends on the ability of the medication to reach the intended areas, and the distribution of medicated particles delivered by pump sprays or metered-dose inhalers has been extensively studied over the years. Several early studies [7–9] demonstrated that spray deposition occurred only in the anterior 1/3 of the nasal cavity, specifically regions of the vestibule, inferior turbinate, and nasal floor. These early studies imply that significant deposition to the middle meatus may not occur; thus standard pump delivery systems may be ineffective for CRS treatment. When volume of medication was increased to 50–100 μL [8], the likelihood of deposition beyond the anterior 1/3 of the nasal cavity was increased. Keyhani and colleagues [10] demonstrated that particle distribution into nasal airstreams depended on location of release with the most optimal delivery to the middle meatus occurring when the release point was anterior and lateral within the naris. This placement may facilitate flow into the middle meatus; however, whether particles deposited actually enter the sinuses themselves remained a question. Hyo et al. [11] determined that the optimal particle size varied with maxillary sinus ostium size. While 3–10 μm was found to be ideal for penetrating the sinus, only 3 % of those particles actually penetrated the sinus. Using a cast model study, Saijo and colleagues [12] demonstrated that both angle of release and particle size played significant roles in penetration of the ostiomeatal complex (OMC) and maxillary sinus following surgery. Computer simulations revealed a 45-degree angle improved deposition over a 30-degree angle and particles 5.63 μm in diameter were significantly more effective than particles 16.37 μm in diameter. Whether 5.63 μm or 16.37 μm, this particle diameter is notably smaller than the average droplet size (50–100 μm) [13] delivered with conventional pump release sprays, and this raises the question as to whether FDA-approved sprays for CRS deliver any significant medication to any of the paranasal sinuses.
Nebulizer Systems
Similar to nebulizer systems for managing asthma and other chronic pulmonary conditions, nebulizers deliver medications to the nose and paranasal sinuses in the form of an aerosolized mist or vapor. Nebulizers may be subdivided into passive-diffusion systems that produce smaller-sized particles that travel via lower velocity in a single direction and vortex-propelled systems that produce larger particles. Commercially available passive-diffusion systems include the SinuNebTM (PARI Respiratory Equipment, Midlothian, VA), which generates particles <5 μm in diameter, relies on inspiration, and is subject to placement and the predicted pathways of nasal airflow. ViaNaseTM (Kurve Technology, Inc, Lynnwood, WA) is an example of a vortex-propelled system that generates particles between 9 and 11 μm in diameter that are inhaled. Both passive-diffusion systems and vortex-propelled systems were studied by Hwang and coworkers [14] using radiolabeled saline. Sinus penetration was noted to be poor with both systems, although the vortex-propelled system showed greater frontal sinus (30 %) and sphenoid sinus (30 %) penetration. Endoscopic sinus surgery did not significantly enhance distribution.
As noted by Hyo and colleagues [11], particles between 3 and 10 μm were theorized to achieve maxillary sinus penetration, while Saijo and coworkers [12] demonstrated that smaller particles (5.63 μm v. 16.37 μm) and higher flow rate had improved maxillary sinus penetration; however, ostial size was noted to the biggest factor in sinus penetration. Therefore, a number of factors call into question the efficacy of many nebulizer systems as a method for treating CRS since particle size may be incompatible with significant sinus penetration and the method of particle generation may result in significant nasal cavity filtering.
Pulsating nebulizers such as RhinoFlowTM (Respironics, Inc., Cedar Grove, NJ) and NasoNebTM (Medinvent, Medina, OH) generate large-sized particles (>10 μm, average 20–30 μm), which are large enough to be filtered by the nasal cavity and, based on previously mentioned studies, may be too large to penetrate the unoperated sinuses. Negley and coworkers [15] evaluated the effectiveness of the RhinoFlowTM system in a small sample of patients without CRS and found inconsistent delivery of Tc99m into the sinuses. Whether surgery enhanced nebulizer delivery was tested by Manes and colleagues [16] who evaluated the NasoNeb nebulizer on five cadaver heads before and after ESS. This trial revealed consistent delivery of aerosolized saline with fluorescein to the middle meatus/ethmoid cavity and sphenoethmoidal recess. After cadavers underwent endoscopic sinus surgery, there was a significant improvement in delivery to middle meatus and to the maxillary sinus and ethmoid cavities. Delivery to the frontal sinus was enhanced by performing an endoscopic modified Lothrop.
Finally, Möller et al. [17] evaluated the distribution of 99mTc-DTPA in 11 patients with CRSsNP before and after endoscopic sinus surgery in patients using the Vibrent nebulizer (PARI Pharma GmbH, Starnberg, Germany), which generates 3.0 μm particles. Deposition in the nasal cavity as well as the paranasal sinuses was measured, and by at least 2 months following surgery, there was a significant decrease in total nasal deposition matched by a simultaneous significant increase in maxillary and sphenoid sinus deposition. An interesting and unexpected finding in their study was that the deposition of particles within the nasal cavity in healthy volunteers did not differ significantly from patients with CRSsNP, suggesting that in patients with CRSsNP, the nasal cavity is still an effective filter. They found evidence of deposition into the maxillary and sphenoid sinuses in patients with CRSsNP, which was also an unexpected finding.
Nebulizer devices vary in technology and delivery technique; however, it would seem that as a group, these devices do not consistently offer a reproducible and reliable means of delivering medications to the sinuses. Although a cadaveric study using the NasoNeb system appears promising, more studies on this device are required before definitive conclusions can be drawn.
Drop Delivery
Otologic or ophthalmic preparations of steroids have been used to treat CRS as well. Delivery of such low-volume drop medications to the sinuses depends heavily on technique, and a handful of studies have looked at the distribution of drops within the nasal cavity and sinuses. Kubba et al. [18] found that betamethasone delivery to the middle meatus was best achieved in the Mygind and “head down and forward position” positions and that the “head back” technique (See Fig. 16.1) resulted in nothing more than nasal floor and nasopharynx distribution. The Mygind position was recommended since it was generally viewed as more comfortable than the “head down and forward” position. The delivery of drops to the middle meatus may not be optimal, however, as evidenced by Homer and coworkers [19] who found that although the average amount of radiolabeled medication in middle meatus pledgets was higher in the patients using drops, it was not more significant than in those using a nasal spray device. In another study, Rudman and colleagues [20] utilized a cone beam scanner to evaluate the distribution of a radiopaque contrast solution via drop delivery when in the vertex-to-floor position. Contrast was not seen in areas superior to the middle turbinate; rather nasal cavity spaces such as the vestibule, inferior meatus, and anterior nasal cavity had the majority of distribution.
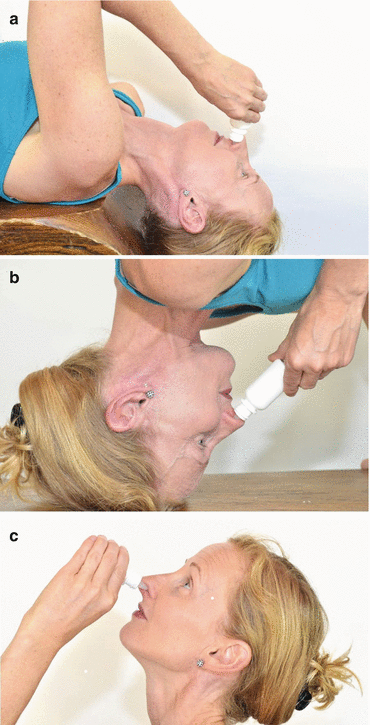

Fig. 16.1
This series of photographs depicts the administration of nasal drops via the Mygind (a), head down and forward position (b), and head-back (c) positions (Photo courtesy of David P. Welch)
Drug delivery via nasal drops appears to be very dependent upon head position, and given the low volume/high concentration of the drug delivered, accurate deposition within the sinuses may provide a viable means for treating some forms of CRS. However, the low volume raises cost issues since otologic and ophthalmic preparations of steroids.
High-Volume/Low-Pressure Bottle Irrigation
Aqueous preparations of steroids can be mixed with saline and used in commercially available bottle irrigation systems. Patients performing medicated irrigations typically add a steroid respule into either 120 or 240 mL of saline solution and irrigate the nasal cavity and sinuses. This may be performed via positive pressure (squeeze bottle) or gravity (neti pot) (See Fig. 16.2). This method of delivery has gained popularity over the recent years because of evidence demonstrating that the irrigant has the widest distribution within the unoperated and operated sinuses when compared to other delivery mechanisms.
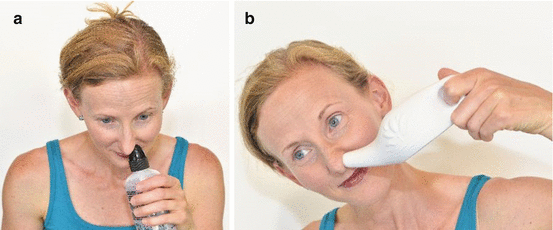

Fig. 16.2
This series of photographs depicts the administration of irrigation via a squeeze bottle (a) and neti pot (b) (Photo courtesy of David P. Welch)
Miller and coworkers [21] evaluated the effectiveness of a nebulizer, an atomizer, a spray, and a bulb in delivering dye to the sinuses in seven post-ESS patients. The bulb syringe was found to be the most effective method of delivery to all sinonasal sites. Similarly, Olson et al. [22] evaluated the distribution of isotonic, nonionic contrast material in eight healthy volunteers using positive-pressure irrigation, negative-pressure irrigation, and a nebulizer. Analysis of posttreatment CT scans demonstrated that positive- and negative-pressure irrigation systems resulted in more significant distribution to the sinuses compared to the nebulizer; moreover, the positive-pressure irrigation method provided the best results. Lam and colleagues [23] compared the effectiveness of spray bottles to irrigation bottles in delivering methylene blue dye to the olfactory regions in eight cadaveric heads. Based on blind review of staining patterns, irrigation bottles demonstrated greater penetration of the sphenoethmoidal recess, superior turbinate, and olfactory region.
Finally, Harvey and colleagues [24] evaluated the effects of ESS on sinus distribution of Gastroview in cadaver heads by using a pressurized spray, a neti pot, and a squeeze irrigation bottle. Distribution of Gastroview was significantly higher in the post-ESS cadaver head with the neti pot performing the best. The pressurized spray demonstrated the poorest sinus distribution. It is important to note, however, that even with extensive sinus distribution, the amount of retained irrigant within the sinuses is approximately 2.5 % [25], making it critical to determine the appropriate concentration or dosing of the delivery.
Based on these studies, the use of a high-volume/low-pressure delivery system such as an irrigation bottle or neti pot allows one to deliver targeted therapy to multiple sinonasal subsites. Since ESS enhances delivery of irrigation bottle content to the sinuses, this represents an important adjunct in the postoperative period.
Biomaterials/Implants
The delivery of topical steroids may also be accomplished through the placement of biomaterials or implants into the sinonasal cavity following surgery. These delivery devices include FDA-approved biomaterials such as mometasone furoate (Propel®, Intersect ENT), which is placed within the ethmoid sinuses during surgery or in the office setting via a delivery catheter, as well as off-label delivery of steroids via temporary, self-absorbing dressings utilized as middle meatus spacers (Gelfoam, XeroGel®, Nasopore®). Biomaterials and implants represent a mechanism for the one-time physician-directed instillation of a topical steroid that is maintenance-free for the patient.
Summary of Methods
There are several options for delivering topical steroids to the nasal cavity and paranasal sinuses, and the choice of delivery method depends on a number of factors. All of these methods are capable of delivering topical steroids to the nasal cavity and sinuses; however, it is clear that some techniques result in more effective delivery. Much of this is dependent upon the device and the operated state of the sinuses. Thomas et al. recently performed an evidence-based review with recommendations on the distribution of topical agents to the sinuses, assessing multiple factors affecting distribution such as delivery device, head position, outcome assessment, anatomy, patient factors, disease states, etc. [26]. The final recommendations based on their pooled assessment include the following:
1.
Sinus surgery increases sinus penetration of topical therapies.
2.
High-volume delivery devices are recommended.
3.
Head position should be down for high-volume delivery devices.
4.
High-volume delivery devices overcome “unfavorable” nasal and sinus anatomy.
Topical Steroids for Chronic Rhinosinusitis
Topical Steroids in Chronic Rhinosinusitis Without Polyposis
Several randomized clinical trials (RCT) have evaluated the use of topical steroids in the management of CRSsNP; however, the recommendations remain unclear, given the variability of these multiple studies. Several RCTs have evaluated topical steroid spray against placebo in the management of CRSsNP [27–32]. Sykes and colleagues [27] compared the outcomes in 50 patients with mucopurulent rhinosinusitis treated with combination dexamethasone/tramazoline sprays with or without neomycin against placebo. Patients receiving placebo sprays did worse, and there was no significant difference in groups receiving the antibiotic. Although patients in both dexamethasone/tramazoline groups improved, the addition of the tramazoline component makes it difficult to ascertain the exact effect of the dexamethasone in this study. Parikh et al. [28] followed 22 patients randomly assigned to 200 mcg of fluticasone spray or placebo and, after 16 weeks, noted no difference between the 2 groups in measured parameters (symptoms, endoscopy scores, acoustic rhinometry, and serology studies). Likewise, Dijkstra and colleagues [29] evaluated the effect of fluticasone 400 mcg or 800 mcg vs. placebo on the recurrence rate of CRS and nasal polyps over a 1-year period following ESS and found no significant differences in outcomes.
On the other hand, Lund and coworkers [30] evaluated the efficacy of budesonide aqueous nasal spray against placebo in a multicenter randomized trial and found that in patients with CRSsNP, budesonide significantly decreased combined symptom scores while at the same time improved sense of smell and peak nasal inspiratory flow (PNIF). Jorissen et al. [31] compared mometasone furoate nasal spray and placebo in wound healing following endoscopic sinus surgery for 6 months and found that although total endoscopic scores did not differ, combination scores did improve in the mometasone furoate group, indicating that mometasone furoate may improve healing. Finally, in a 12-week RCT of a bidirectional delivery device (OptiNose) administering fluticasone propionate or placebo, patients with CRSsNP demonstrated improvements in all parameters measured (endoscopy scores, PNIF, symptoms VAS, and RSOM-31scores). Magnetic resonance imaging (MRI) scores did not differ significantly.
In a comprehensive systematic review and meta-analysis, Kalish et al. [33] reviewed the evidence on the use of topical steroids in patients with CRSsNP. Six of the eight trials demonstrated that topical steroids significantly improve symptoms. However, as noted in the review, the studies used different outcome measures, precluding definitive conclusions on the efficacy of topical steroids for CRSsNP other than to say that topical steroids are low-risk interventions that may be beneficial. Snidvongs et al. [34] expanded on this in a more formalized Cochrane Review and evaluated the outcomes in patients without polyps who were treated with topical steroids. Pooled data from ten RCTs in this analysis revealed that patients with CRSsNP treated with topical steroids had significant improvements in overall symptoms as well as objective response to therapy, and this was not influenced in their subgroup analysis by the presence or absence of surgery. A subgroup analysis showed some benefit to using direct sinus application over nasal application of the steroid. This review was unable to determine if topical steroids in patients with CRSsNP resulted in significant radiographic changes, endoscopic scores, or disease-specific quality of life, however. The authors’ conclusions were that adverse effects were infrequent and that topical steroids should be utilized as part of a comprehensive management of CRSsNP.
Steinke et al. [35] performed a pilot study in patients with hyperplastic CRS to assess the effects of 0.5 mg budesonide in 100 mL saline. Over 3 months, patients experienced a decrease in symptoms as well as improvement in endoscopy and Lund-MacKay CT scores. Snidvongs and colleagues [36] also evaluated the efficacy of steroid irrigations on CRSsNP. Following surgery, patients received either 1 mg budesonide or 1 mg betamethasone, and significant improvements in baseline compared to posttreatment were noted for SNOT-22 and endoscopic scores.
A recent evidence-based review with recommendations was performed by Rudmik et al. [37] who proposed recommendations after evaluating the data on the use of topical steroids in patients with CRSwNP and CRSsNP. For standard therapies (i.e., nasal steroid spray), the aggregate quality of evidence was given a grade of A. Main benefits were improved symptoms and endoscopic appearance as well as polyp size reduction. Given the lack of significant side effects and adverse events, the use of standard topical steroids was given a “strong recommendation” for routine use with a preponderance of benefit over harm. For nonstandard therapies (irrigations, drops, etc.), the aggregate quality of evidence was given a grade of C. The benefit was reduced ostial stenosis and reduced use of systemic steroids. A recommendation of “option” was given in these cases due to heterogeneity and paucity of studies.
Topical Steroids in Chronic Rhinosinusitis with Polyposis
Topical steroids have long been used to treat nasal polyps [38], and several early RCT studies [39–46] demonstrate consistently that use of topical steroid sprays (when compared to placebo) in patients with CRSwNP results in improved symptoms and quality of life and reduction in polyp size. These studies encompass a wide variety of steroids as demonstrated in Table 16.1; however, none of these studies compare one topical nasal steroid spray to another in the management of CRSwNP. Since the extent of improvement among outcome measures varies from study to study, no conclusion can be drawn as to whether one topical nasal steroid spray is superior to another. Nevertheless, there is some evidence that demonstrates that higher doses of steroid sprays may result in better outcomes [47]. The efficacy of nasal sprays depends in many ways upon compliance and technique; therefore, a cross-hand spray (left hand sprays the right nostril) is frequently advocated, and patients are often reminded to make administration part of a daily ritual.
The utilization of steroid drops has the potential to provide patients with an easily portable delivery system that may work as effectively as nasal sprays in the management of CRSwNP. Unlike nasal sprays which rely on a slightly head-down position, correct placement of nasal drops relies heavily on the Mygind or “head down and forward position” position. A number of studies have evaluated the efficacy of steroid drops. Penttila and coworkers [48] randomized 142 patients with bilateral polyps to receive either fluticasone 400 mcg nasal steroid drops b.i.d. or placebo. After a 12-week treatment period, patients receiving fluticasone experienced significant reduction in polyp size and significant improvements in nasal parameters such as PNIF. Similar findings were reported by Chalton et al. [49] with betamethasone drops and by Aukema and colleagues [50] using fluticasone drops. In contrast, Ehnhage et al. [51] evaluated the effect of ESS on asthma in patients with polyps, and although surgery did benefit asthmatics, an evaluation of fluticasone drops showed no benefit in disease outcomes when compared to placebo. DelGaudio and Wise [52] retrospectively evaluated dexamethasone, prednisolone, and ciprofloxacin/dexamethasone drops in patients undergoing revision ESS at high risk for polyp recurrence and found patients to have decreased ostial stenosis and decreased need for revision surgery. Therefore, while topically placed steroid drops do offer a beneficial alternative therapy for patients with CRSwNP, the likelihood of success may depend upon disease and surgery status and heavily upon head position during administration.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree