Fig. 7.1
(a) An isolated fungus ball may appear as nonspecific sinus opacification on a CT scan with bone window settings. Close inspection of the right maxillary sinus suggests some hyperdensities, but the finding is not dramatic. (b) This CT scan (now at soft tissue windows) of the same patient seen in (a) demonstrates hyperdensities that are characteristic of an isolated fungus ball. Intraoperative findings confirmed the diagnosis of a right maxillary fungus ball
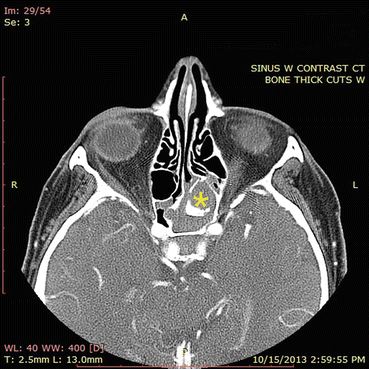
Fig. 7.2
Sinus CT angiogram demonstrates hyperdensity within an opacified left sphenoid sinus. This appearance is consistent with isolated sphenoid fungus ball
MRI findings of a fungus ball are somewhat less specific than CT findings. On MRI, a fungus ball may produce various signal intensities that reflect the extent of hydration of the sinus contents. At its extreme, a fungus ball will produce loss of signal on both T1 and T2 MRI modalities, and thus, in this circumstance, a long-standing fungus ball will produce no signal on MRI and will mimic the appearance of a normally aerated sinus.
Treatment
Treatment primarily consists of surgical debridement of the affected sinus cavity (Fig. 7.3), with relatively low rates of recurrence thereafter. Postoperative sinus cavity fungal balls may be amenable to in-office debridement or treatment with conservative nasal irrigation measures, although frequently more aggressive debridement measures under sedation are required. Medical therapy targeting this disease entity is largely ineffective.
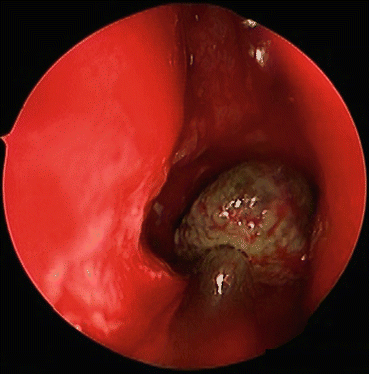

Fig. 7.3
During surgical removal, fungus balls appear as dense discrete balls of fungal elements and related debris, as seen in this endoscopic view of a right maxillary fungus ball that has been displaced into the middle meatus for removal
Although it is a relatively straightforward decision to operate on a symptomatic fungal ball, an incidentally found, clinically quiescent fungal ball might give the clinician pause, especially in a patient with severe comorbidities and anesthesia risks. While observation is always a viable option, it should be noted that, in the immunocompromised host, progression of fungal ball to invasive fungal sinonasal disease has been reported [11]. This suggests the need for aggressive management when this entity is identified in someone with or at risk for immunosuppression. This potential for progression also justifies surgically treating the asymptomatic healthy individual, as the disease is likely to persist indefinitely over the course of one’s lifetime and the risk for immunocompromising illness always exists. Furthermore, an opacified sinus may also harbor an occult neoplasm. Thus, surgical intervention is warranted for both asymptomatic and symptomatic fungal balls that are noted on imaging.
Allergic Fungal Rhinosinusitis
Allergic fungal rhinosinusitis (AFRS), originally described in 1983 by Katzenstein et al. [12], represents a subclass of CRS that accounts for up to 10 % of CRS cases in the United States [12, 13]. It most commonly affects young, immunocompetent individuals with fungal atopy, is mostly unilateral, and has a geographic predilection to both humid and arid environments, such as those seen in the southern United States, Middle East, Australia, and Africa [13–15].
Diagnostic Criteria
Consensus clinical guidelines, based on widely accepted criteria proposed by Bent and Kuhn, define AFRS by the following characteristics: (1) presence of nasal drainage, nasal obstruction, decreased sense of smell, and/or facial pressure for 12 weeks, (2) mucin within the sinus cavity containing fungal hyphae and degranulating eosinophils, (3) endoscopic evidence of inflammation within the sinus cavity, (4) CT or MRI findings consistent with chronic rhinosinusitis, (5) evidence of fungal-specific IgE by skin prick or serum IgE testing, and (6) no evidence of invasive fungal disease [16, 17].
Fungal culture, though contributory to the diagnosis if positive, is variably sensitive; therefore, the histologic appearance of eosinophilic mucin with fungus remains the more reliable indicator of AFRS [18]. Typical histologic findings include branching, noninvasive fungal hyphae, lamellated sheets of eosinophils, and elongated eosinophilic breakdown products known as Charcot-Leyden crystals (Fig. 7.4). Endoscopic evidence of inflammation typically shows polypoid mucosa in proximity to thick, highly viscous discolored secretions (tan, brown, and/or green) (Fig. 7.5).
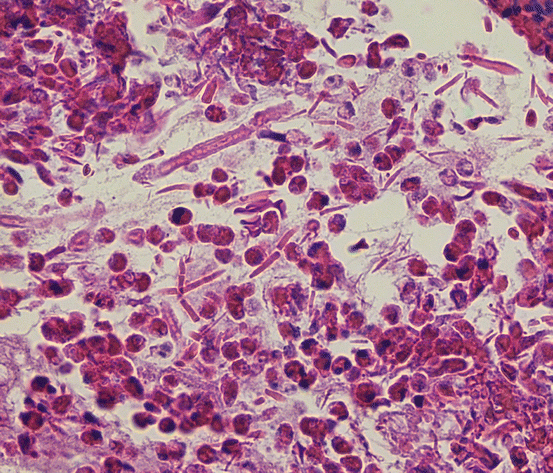
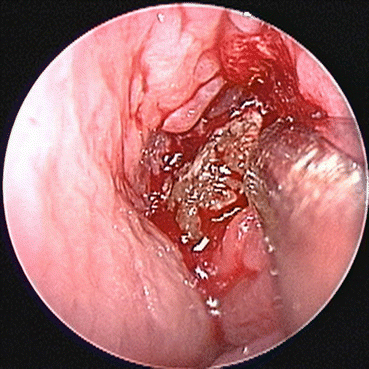

Fig. 7.4
Allergic fungal rhinosinusitis is characterized by eosinophils, fungal hyphae, and Charcot-Leyden crystals, as seen here (hematoxylin and eosin, 400×)

Fig. 7.5
Allergic fungal rhinosinusitis is characterized by the presence of eosinophilic debris, demonstrated in this endoscopic image
Presentation
Patients with AFRS present with symptoms of long-standing, difficult-to-treat CRS. Major sinonasal symptoms include congestion, drainage, nasal obstruction, etc. Often patients with AFRS will report nonspecific symptom of congestion with minimal quality of life impact. In rare instances, a patient with AFRS will present from proptosis due to otherwise asymptomatic expansion of adjacent sinus cavities with eosinophilic debris. Patients tend to be young adults and adolescents, and aspirin-exacerbated respiratory disease is rare in the AFRS patient cohort. These patients are considered immunocompetent by conventional testing methods. In fact, there is often some evidence of exuberant inflammatory response, as indicated by an elevated total serum IgE, which is typically elevated between 500 and 4,000 kUA/l [19]. Some AFRS patients will also have asthma. AFRS not uncommonly occurs asymmetrically; although up to 50 % of cases may show bilateral disease at presentation, 80 % of cases have a strong unilateral predominance [20]. If the AFRS is advanced, it may expand the bone, leading to orbital deformities and proptosis. Advanced AFRS may also cause diplopia and visual loss, due to compression of critical structures from sinus expansion. Nasal endoscopic examination typically demonstrates nasal polyps, as well as thick, tenacious, light tan/brown or green secretions known as eosinophilic mucin (Fig. 7.5).
Characteristic CT findings include expansion of involved sinuses with demineralization of cortical bone. Dual-density secretions are seen on CT, which are related to the accumulation of heavy metals and calcium salt precipitate within the mucin (Fig. 7.6). MRI often shows isointense or hypointense secretions on T1-weighted imaging and hypointense secretions on T2-weighted imaging related to the dehydrated state of mucin; adjacent mucosa enhances on both modalities (Figs. 7.6 and 7.7) [20, 21].
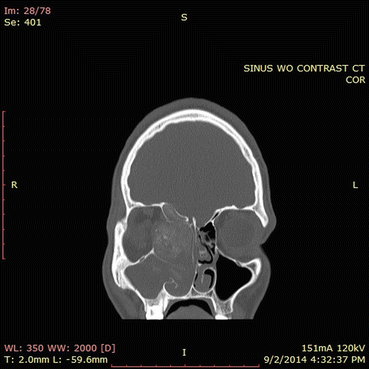
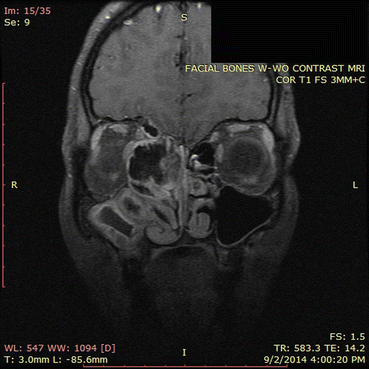

Fig. 7.6
In this CT scan, the right ethmoid sinuses contain an expansile process. Note the hyperdensities present in the right ethmoid sinuses and in the right maxillary sinus to a lesser extent. The orbital wall is eroded and the sinus process is compressing the orbital contents. The ethmoid roof has also been eroded. This CT scan illustrates the classic findings of allergic fungal rhinosinusitis

Fig. 7.7
This MRI image corresponds approximately to the same region as the CT scan in Fig. 7.6. The areas of signal dropout (which appear black in the image) correspond to the hyperdensities seen on the corresponding CT scan and represent areas of eosinophilic fungal debris. Imaging of adjacent orbital and intracranial structures confirm the absence of invasion by the sinus process. This MRI illustrates classic findings of allergic fungal rhinosinusitis
Etiology
The pathophysiology of AFRS remains unclear. Dysregulated T helper 2 (Th2) responses appear to be a major driver of the pathogenic pathway but the inciting actors and specific mechanisms eliciting the hyperactive Th2 immune cascade are still the subject of ongoing investigation.
Originally, AFRS was thought to represent purely an IgE-mediated type I hypersensitivity reaction to fungus in the sinonasal cavity. By definition, AFRS has a predominance of fungi within accumulated eosinophilic mucin in the affected sinuses; this association strongly suggested an etiologic role of fungi. Early observations on AFRS patients demonstrated a correlation between the presence of fungal allergy in patients with AFRS and CRS patients without AFRS. In one report, 8 patients with AFRS who had sinus cultures positive to Bipolaris also showed Bipolaris-specific IgE and IgG serum elevations and Bipolaris reactivity on skin prick testing; 80 % of CRS patients were negative on both serum and skin prick testing [18]. This fungal-specific IgE elevation suggests a pathologic contribution through the adaptive immune response which is further supported by elevated T helper 2 cytokines (i.e., IL-4, IL-5, and IL-13) and eosinophil levels within the sinonasal mucosa of AFRS patients [22]. Additionally, an in vitro challenge of peripheral blood mononuclear cells from patients with AFRS to common etiologic fungal antigens incited the secretion of Th2 cytokines from these cells; this response was not elicited in mononuclear cells from healthy controls exposed to the same antigens [23]. These aforementioned studies support the conclusion that AFRS patients have fungal hypersensitivity and immunologic memory to fungi, but a causal relationship still has not been established.
Abnormalities in fungal-specific IgG have also been reported in AFRS patients. Bipolaris-specific IgG elevations have been identified in Bipolaris-positive AFRS patients [18]. Fungal-specific IgG elevation suggests a possible role of the Gel and Coombs type 3 hypersensitivity response, whose first step is immune complex formation between specific IgG to the target antigen. In support of presumed mechanism, fungal-specific IgG3 was the only marker that was distinctly elevated and thereby differentiated patients with eosinophilic CRS (including those that fit criteria for AFRS) from patients with other forms of CRS and from patients with allergic rhinitis only [24].
Defects in innate immunity have also been confirmed in AFRS. Fungal spores are ubiquitous in the environment and are considered immunologically inert; therefore, spores must breach the innate immune system and germinate into hyphae in order to incite an inflammatory reaction [25]. Structural abnormalities in airway epithelium may contribute to this process, with the subsequent defective clearance of spores fostering the germination of the fungal spore into hyphae [19]. This mechanism is the presumed cause of allergic bronchopulmonary aspergillosis, a pulmonary condition whose features are similar to AFRS. Additionally, it seems plausible that additional host factors such as a defect in innate immunity and/or mucosal structural abnormality must exist in order for the distinct pathology of AFRS to develop, since fungal atopy usually does not lead to the development of AFRS. The finding of an increased incidence of class II major histocompatibility complex (MHC) HLA-DQB1*0301 and *0302 in patients with AFRS implicates such defects in innate immunity playing a role in the disease process [26].
Once the innate immune defense system is infiltrated, allergic antigens such as fungi may utilize proteases to incite inflammation. Proteases have been shown to have activity that accompanies the antigenic activity of common allergens including mold [27, 28]. Additionally, protease exposure has been linked to allergic respiratory disease through occupational exposure studies [29]. The association of fungal protease activity and allergic airway disease is further supported by studies in which intranasal proteinase exposure in mice without prior sensitization was able to elicit pulmonary changes consistent with those seen in the classic model of allergic asthma [28]. The molecular signaling associated with fungal protease activity and allergic airway disease has been linked to at least two receptors: toll-like receptor 4 (TLR4) and protease-activated receptor 2 (PAR-2) [30, 31].
Another proposed etiologic factor in CRS (and AFRS) involves bacterial superantigen. Superantigen is known to have the ability to incite massive immune reactions through the nonspecific, polyclonal activation of T cells, and Staphylococcus aureus, recognized as a prodigious superantigen producer, is commonly found in the sinuses of individuals afflicted with AFRS [32, 33]. Recent work has shown that specific IgE to staphylococcal antigen enterotoxin (SE-IgE) is an independent risk factor for asthma in a concentration-dependent manner [34]. This same effect was not seen with specific IgE to aeroallergens. Additionally, the presence of SE-IgE was associated with elevated total IgE, representing possible polyclonal IgE production in response to the superantigen [34]. Interestingly, non-atopic asthmatics (negative skin prick testing and/or serum IgE testing against aeroallergens) were shown to be positive for SE-IgE [34, 35].
As in asthma, mechanisms of inflammation in AFRS may be similarly propagated through the staphylococcal enterotoxin. The Th2 immune response to fungus could foster the environment for Staphylococcus aureus, as inflammation hinders the ability of the innate immune system and the epithelial barrier to adequately defend against pathogens [36]. Subsequent polyclonal B- and T-cell activation induced by the superantigen would lead to ongoing inflammation [37]. In support of this, selective IgE was present in 16 of 17 patients with AFRS and there was a significant and strong correlation of SE-IgE to total IgE in these patients; this same effect on total IgE was not seen with specific IgE to fungal antigens [36]. However, co-expression of SE-IgE and selective IgE to fungal antigen did significantly elevate total IgE when compared to patients with CRSwNP. This suggests a possible synergistic relationship between fungus and Staphylococcal aureus. The recent detection of biofilms containing both fungi and Staphylococcal aureus on electron microscopy in the AFRS patients supports this hypothesis [36].
Recently, attention has been directed toward epithelial cell-derived cytokines driving the local Th2 response in both AFRS and CRSwNP. In addition to their barrier function, these pseudostratified epithelial cells of the sinus mucosa are able to incite a local Th2 immune reaction to various environmental triggers, including fungi [38]. Important mediators released by the epithelial cells include IL-25, IL-33, and thymic stromal lymphopoietin; recent studies have shown these actors to lead to increased IL-13 production and mast cell activation, thus propagating the Th2 pathway [38–40].
Treatment
AFRS treatment starts with appropriate diagnosis. Clinical symptoms, coupled with endoscopy and CT findings, will often yield a tentative AFRS diagnosis, but definitive diagnosis requires histopathologic analysis of mucin obtained at the time of surgery. In relatively rare instances, AFRS may only be detected during surgery for CRS refractory to medical treatments. As with other forms of FRS, endoscopic sinus surgery (ESS) provides platforms for diagnosis, treatment, and long-term management. It must be emphasized that surgery alone is rarely sufficient treatment; almost all patients will require ongoing treatment with oral or topical corticosteroids, which are potent ways to reduce the inflammatory burden in affected sinuses.
As previously stated, the first goal of ESS is to achieve a diagnosis. In addition, during ESS, the surgeon must remove all eosinophilic debris, which presumably contains inciting fungal antigens and other potential contributing triggers from all involved sinuses [15]. Failure to remove all debris leaves a focus that will drive persistent sinus inflammation and lead to surgical failure. The final goal of surgery is to create open sinus cavities so that patients can perform postoperative irrigations. Through irrigations, patients may deliver additional medications directly to the mucosa and space of the sinuses, and the irrigations themselves serve as debridement by washing out elements of fungus and mucin. After effective surgery, the surgeon may also easily visualize the state of the sinus mucosal health and perform additional office-based debridement of the open sinus cavities [15].
In preparation for surgery, many surgeons will institute systemic steroids (prednisone, at a dose 0.5/kg/day or more) for 3–5 days (or longer) preoperatively. The goal of this treatment is to reduce the inflammatory burden and thus reduce bleeding. It should be noted that the administration of preoperative systemic steroids may obscure an AFRS diagnosis, by reversing many of the findings of AFRS. In addition, some surgeons may treat with empiric or culture-directed systemic antibiotics, although the impact of such treatment has never been demonstrated.
Although the inflammatory burden is great, the surgeon must follow established principles of functional surgery, with care taken for preservation of normal structures. In advanced cases of AFRS, the eosinophilic debris will cause a massive expansion of the paranasal sinus volume, which may contain large amounts of thick, retained secretions that will often clog suctions easily. Thus, the surgeon must use meticulous techniques to remove all eosinophilic material. Intraoperative irrigation may help dislodge retained secretions. Clumps of eosinophilic material may often be found adjacent to areas of bony dehiscence; therefore, any surgical manipulation in these areas requires extra attention to avoid inadvertent injury to the skull base and orbit.
Destructive sinus procedures should be avoided, because in general, they are not necessary, and in some cases, they may complicate long-term care. In AFRS patients, the frontal recess access is typically expanded by the mucin, obviating need for extensive bone removal at the boundaries of the frontal recess. Frontal sinus obliteration procedures carry the risk of burying viable fungal elements in the obliterated cavity and thus may set up long-term complications. Even the presence of erosion of the bony sinus walls does not mandate sinus obliteration [41].
Comprehensive AFRS treatment includes a prolonged period of postoperative care. During the postoperative period, the surgeon will correlate symptoms against endoscopic findings and then use this information to guide ongoing medical treatment. The AFRS endoscopic staging system classifies the postoperative sinus cavities into 4 distinct stages: no evidence of disease (stage 0) (Fig. 7.8a), edematous mucosa with or without mucin (stage I) (Fig. 7.8b), polypoid mucosa with or without mucin (stage II) (Fig. 7.8c), and polyps with fungal debris (stage III) (Fig. 7.8d). The general principle of treatment is that medical treatments should only be reduced when the endoscopic examination is at stage 0 for at least 3–4 weeks. In addition, it is probably advisable to increase or at least maintain current treatment for patients with higher stages of postoperative disease, even if the patient is asymptomatic [42].
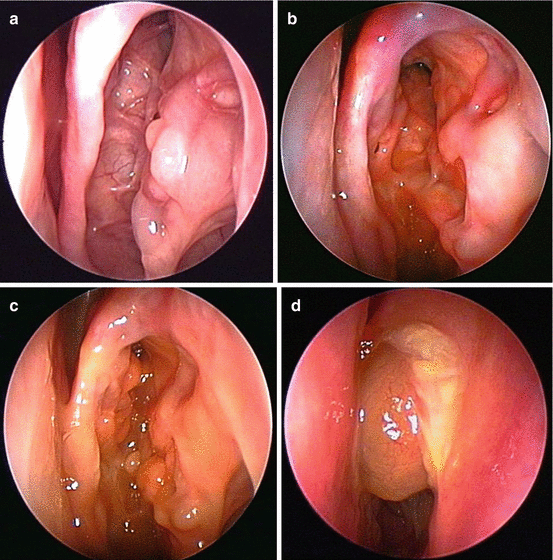

Fig. 7.8
(a) Stage 0 of the endoscopic staging system of allergic fungal rhinosinusitis is a normal exam, without any evidence of active mucosal inflammation. (b) In stage I of allergic fungal rhinosinusitis, the mucosa shows signs of edema with or without the presence of eosinophilic mucin. (c) In stage II of allergic fungal rhinosinusitis, the mucosa has become polypoid. Eosinophilic mucin may or may not be present. (d) In stage 4 of allergic fungal rhinosinusitis, the involved sinuses are filled with polyps and eosinophilic debris
In addition to surgery, medical therapy targeted at the chronic inflammatory response is needed. Despite recent appreciation of activated immunologic pathways and the role of various environmental triggers in AFRS, steroids remain the cornerstone in medical treatment. Steroids, both topical and oral, are critical for treatment success. Multiple prior studies have shown improved symptom scores and lower polyp recurrences in postoperative AFRS patients who were treated with perioperative oral corticosteroids and/or prolonged topical steroids [43–49]. Despite this, chronic oral steroid use is limited by well-documented side effects, including but not limited to osteoporosis, osteonecrosis, diabetes, glaucoma, cataracts, weight gain, hypertension, and adrenal suppression. Fortunately, topical steroids, even when administered via irrigations, appear to have a positive safety profile in postoperative patients [48, 50].
Surgeons use many different protocols for postoperative corticosteroid treatment. Unfortunately, rigorous studies have not confirmed the benefits of one approach over another. In general, prednisone should be administered to AFRS in the immediate postoperative period at a dose 0.5 mg/kg/day; these steroids can be then tapered over 2–3 weeks or longer, depending on the endoscopic exam and symptoms. Many patients will require at least several weeks of systemic prednisone for stabilization of mucosal health. Before stopping the prednisone, most surgeons will also add in high-dose topical steroids, which are continued for longer periods. Standard formulations of nasal steroids delivered as nasal sprays can be used on BID-TID schedule (2–3 times the FDA-approved doses for allergic rhinitis). Other options include budesonide (0.25–0.5 mg BID-TID), fluticasone (3 mg BID-TID), and betamethasone (0.75 mg BID-TID), delivered via low-pressure/high-volume irrigations (200–250 ml of saline) or various commercially available sinonasal nebulizers (10–20 ml of saline). Budesonide is commercially available for nebulization for asthma treatment and thus may be purchased through large pharmacy chains and neighborhood pharmacies. Both fluticasone and betamethasone may be available from compounding pharmacies. Of these three agents, betamethasone probably has the highest degree of systemic absorption.
Immunotherapy (IT) has also been recommended for AFRS treatment. Early studies, using relatively dilute concentrations of fungal antigen, showed improved outcomes over a 3–5-year period [51–54]. Benefit has also been shown when begun in the immediate postoperative period, with lower rates of disease recurrence and revision surgery in AFRS patients who received IT after surgery [55]. Concern over delayed local reactions and immune complex formation using fungal IT dampened initial enthusiasm, but recent studies have shown no evidence of this using high-dose fungal IT over prolonged follow-up periods, allowing future use and ongoing study of fungal IT in AFRS patients [56, 57].
AFRS exacerbations are often heralded by acute bacterial sinus infections, which, if untreated or undertreated, trigger the inflammatory cascade of AFRS. Thus, acute exacerbations require prompt evaluation. Treatment with culture-directed antibiotics, often coupled with increased steroid treatment (either topical or systemic), can reverse the exacerbation and prevent its progression to an advanced AFRS stage. AFRS has been associated with Staphylococcus aureus, and some otolaryngologists will empirically add topical anti-staph agents to the patient’s postoperative regimen [33]. Mupirocin irrigations may be prescribed as a matter of routine or added later based upon culture results, since mupirocin nasal irrigation targets both planktonic and biofilm forms of Staphylococcus aureus [58]. Despite theoretical benefits, a recent review on topical therapy in CRS highlighted the need for further study of high-volume antimicrobial irrigations based on lack of currently available evidence [59]; however, formal studies of topical antibiotics for AFRS exacerbations have not been performed.
Topical antifungal agents have also been proposed for AFRS treatment, but studies are sparse. Several studies, though not focusing directly on AFRS, have examined the role of antifungal irrigations in CRS in general. A meta-analysis of these studies did not show any benefit from amphotericin B irrigations in CRS patients [60]. A Cochrane review came to a similar conclusion [61]. In general, these studies were poorly designed and included diverse patient populations without the exclusive study of only AFRS patients. Other potentially confounding variables in prior studies include the possibility of inadequate antifungal agent dosing and suboptimal delivery. In light of these deficiencies, it is conceivable that topical antifungal therapy could still prove to be efficacious in an appropriately selected patient population. Ultimately, further study is necessary before conclusions can be drawn regarding its use in AFRS.
Systemic antifungal therapy is also an appealing option for AFRS treatment, but no rigorous studies have been performed in this patient group. A systematic review of this treatment noted that both itraconazole and ketoconazole may have benefit in patients with CRS, but the vast majority of studies were poorly designed uncontrolled case series [61, 62]. A Cochrane review came to similar conclusion [62].
The use of advanced biologics in the treatment of AFRS have not been studied, although there have been a few reports of their use in the general CRS population. An underpowered study of anti-IgE therapy found no objective or symptomatic improvement in CRSwNP patients treated with this novel agent [63]. In another study, anti-IL-5 showed significantly decreased polyp size and less sinus opacification in CRSwNP patients 1 month after treatment compared to CRSwNP patients who did not receive anti-IL-5 therapy [64]. Based on recent findings regarding epithelial derived cytokines, future potential targets may also be directed at IL-25, IL-33, and thymic stromal lymphopoietin among others in the treatment of AFRS and CRS in general [65].
Invasive Fungal Sinusitis
Histologic Considerations for Invasive Fungal Rhinosinusitis Diagnosis
Because of its aggressive nature, early and accurate diagnosis of invasive fungal rhinosinusitis (IFRS) has important implications for treatment selection and eventual outcomes. The priority issue is determination of the presence or absence of fungal invasion into the tissue. Frozen section pathology is the only way to definitively assess for the fungal invasion at the time of surgery. Of course, clinical cues, including the absence of bleeding from affected tissue, also may guide the clinician to an accurate diagnosis. Culture, which is the gold standard for fungus speciation, does not always successfully yield fungal growth and requires days (or longer) for a definitive result.
Microscopic study of fungal morphology can be used to identify the fungus at genus or even supra-genus level. Among the causes of IFRS, fungi from the Mucoraceae family tend to be the most aggressive. As a result, its presence on frozen section strongly suggests the need for more aggressive surgical debridement, although intraoperative findings of tissue viability should primarily guide the extent of surgery.
A more important benefit of microscopic analysis is that antifungal therapy may be more effectively tailored to the specific offending genus [66]. Specifically, amphotericin B and posaconazole are both considered effective therapy for fungi from the Mucoraceae family, which includes Absidia, Apophysomyces, Mucor, Rhizomucor, and Rhizopus. In contrast, voriconazole, which is first-line therapy for IFRS due to Aspergillus species, has no anti-Mucoraceae activity. While amphotericin B is ideally avoided due to its side effect profile including nephrotoxicity and severe infusion reactions, it fortunately does have activity against both types of invasive fungi, and thus amphotericin B is often the ideal antifungal agent in all cases of IFRS, until fungal speciation is confirmed [67].
Important “buzzword” morphologic characteristics of Mucoraceae include “ribbonlike,” “broad based, aseptate,” and “90° (right-angle) branching hyphae.” The Rhizopus species are the most common causes of acute, invasive fungal sinusitis; however, microscopic exam is sometimes the only indication of its presence, given that there is frequently a lack of growth in culture. Silver staining is often only slightly positive or negative in mucormycosis, so a specimen with poorly staining hyphae should alert the pathologist to the possibility of mucormycosis [68]. Aspergillus also has characteristic morphology; Aspergillus species are typically septated hyphae with acute-angle (45°) branching patterns.
In contrast to the fungal species associated with IFRS, various phaeohyphomycotic infections, including AFRS, are associated with dematiaceous fungi (including Alternaria, Curvularia, and Bipolaris species) [66]. An important histologic consideration of this fungal class is that hyphae may be enlarged and globose in shape and are sometimes misidentified as yeast or fungal spores. Nonetheless, it should be possible to distinguish these fungi from more aggressive fungi.
In regard to identification techniques, the primary aim of histopathologic analysis is to highlight the fungal cell wall. Doing so allows fungus to be differentiated from human tissue and its morphology can in turn be better evaluated. While both a pathology and a microbiology lab can perform direct examination of the specimen, one should not rely on gram stain analysis in a microbiology lab to rule out the presence of a fungus. Gram reagents stain fungal cytoplasm, but they do not stain the fungal cell wall. Rapidly growing mold often lacks concentrated areas of cytoplasm due to its transport out to growing tips; with sparse cytoplasmic staining and invisible cell walls, fungus can be missed [66]. More importantly, even if present, invasion cannot be assessed on mycology smears. Thus, formal histologic analysis is always important whenever IFRS is considered based upon clinical presentation.
Common methods used in the histology lab include the hematoxylin and eosin stain, which consists of basic and acidic dyes that stain nuclei dark and extracellular and cytoplasmic material pink. While this method is occasionally sufficient to identify Rhizopus and Aspergillus




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree






