Fig. 27.1
Transnasal view of the left sphenoid ostium showing its position medial to the superior turbinate
An array of surgical approaches have been described for optimal management of inflammatory and neoplastic processes involving the sphenoid sinus.
Introduction
Sphenoid sinus surgery is an important component of the management of inflammatory sinus disease, as well as integral to the approaches to the sellar and parasellar regions for skull base lesions. Given this dual perspective, the surgeon must carefully appraise the surgical goals in the setting of the patient’s anatomy and pathology. Successful surgery requires these considerations, in addition to knowledge of the variable anatomy and anatomic relationships in the region. While complications can occur in any surgical procedure, complications in this delicate region can be devastating and, as with all complications, are best managed with prevention. If encountered, rapid identification and initiation of further diagnostic and/or therapeutic measures is critical.
The embryologic development of the sphenoid sinus typically begins in the third to fourth month of fetal life. It develops from an invagination of the nasal cavity mucosa into the cartilaginous nasal capsule, termed the “cartilaginous cupolar recess of the nasal cavity” [1]. This invagination originates from the posterior ethmoid and does not actually come in contact with the sphenoid bone as it is separated by a cartilaginous plate [2]. This cartilage is resorbed over the first few years of life, allowing the sphenoid sinus to progress into the sphenoid bone. Pneumatization of the sphenoid sinus typically begins at age 1, with the most rapid growth present between 3 months and 5 years of age. Adult size is reached by approximately 12 years of age [3].
The degree of pneumatization of the sphenoid sinus can vary widely and is most often described with respect to orientation in the sagittal plane, termed conchal, presellar, sellar, and postsellar. These terms are defined by pneumatization extending posterior to a vertical line drawn through the tuberculum sellae [1, 2]. Historically, a plain radiograph was used to evaluate this degree of pneumatization leading to the Hamberger classification system of conchal, presellar, and sellar [4]. In a more recent computed tomographic (CT) study assessing the bony configuration in 296 patients, Hamid et al. noted that 2 % had conchal, 21 % had presellar, 54.7 % had sellar, and 22.3 % had postsellar pneumatization [5]. While sphenoid pneumatization in the sagittal plane is of critical importance and drives the nomenclature, aeration into the surrounding bony processes including the greater and lesser sphenoid wings, anterior clinoid processes, pterygoid processes, and palatine bones must also be critically appraised as increasing aeration results in further definition of the surrounding neurovascular structures in the sphenoid walls [2].
The sphenoid bone consists of the following components, each with important neurovascular anatomic relationships: body, lesser wings, greater wings, and pterygoid processes with the medial and lateral pterygoid plates. The body of the sphenoid bone contains the sphenoid sinus and contacts the pituitary fossa, cavernous sinuses with cavernous segments of the internal carotid artery, brainstem with vertebrobasilar arterial system, and nasopharynx on the superior, lateral, posterior, and inferior surfaces, respectively. The greater and lesser wings contribute to the superior orbital fissure, transmitting the oculomotor, trochlear, ophthalmic, and maxillary divisions of the trigeminal nerve and abducens nerves. Of note, the greater wing forms a large portion of the middle cranial fossa and lateral orbital wall, while the lesser wing forms the posterior portion of the orbital roof. The optic nerves are transmitted through the optic canals, which are located superior to the superior orbital fissure and separated from this space by the optic strut, which in the sphenoid sinus is consistent with the lateral opticocarotid recess. At the junction of the greater wing and body, the foramen rotundum transmits the maxillary division of the trigeminal nerve, the foramen ovale transmits the mandibular division of the trigeminal nerve, and the foramen spinosum transmits the middle meningeal artery [6].
Surgical Anatomy of the Sphenoid Sinus
The sphenoid sinus drains into the sphenoethmoidal recess through its natural ostium which is located on the face of the sphenoid. This natural ostium is typically located approximately 1–1.5 cm above the posterior choana and sphenoid sinus floor, situated between the posterior septum and the medial surface of the superior turbinate [2]. This relationship of the natural sphenoid ostium being located medial to the superior turbinate is a guiding principle in safely approaching the sinus. However, in addition to this landmark, other helpful relationships include the face of the sphenoid located at approximately the same depth as the posterior wall of the maxillary sinus (~7 cm from the nasal spine), with the natural ostium at the approximate height of the roof of the maxillary sinus. These relationships emphasize the importance of visualizing the maxillary sinus during sinus surgery to assist with the localization of the suspected position of the natural sphenoid sinus ostium.
Within the sphenoid sinus, there are multiple bony landmarks and walls present with their appearance highly dependent upon the degree of sphenoid bone/sinus pneumatization. The roof of the sphenoid sinus antrum is termed the planum sphenoidale. In well-aerated sinuses, the posterior aspect of the sphenoid sinus will reveal the sella turcica, or the bony covering over the pituitary gland. Just inferior to the sella turcica is the clival recess, which is a portion of the middle third of the clivus. The sphenoid rostrum, or keel, articulates anteriorly with the vomer to form the face and the floor of the sphenoid sinus [2]. If the pterygoid processes (fused portion of the pterygoid plates) have been pneumatized, these extensions of the sphenoid are deemed lateral recesses (Figs. 27.2 and 27.3). Typically, there is a bony indentation at the intersection of the optic nerve and carotid artery located at the posterior-superior aspect of the sphenoid sinus antrum deemed the opticocarotid recess. It should be noted that the optic nerve may be dehiscent in 12.5 % of cases, while the carotid arteries have been found to be dehiscent in as many as 19.5–23 % of cases [7, 8]. Given the highly variable anatomy and very close proximity to vital intracranial and neurovascular structures, careful preoperative planning is of the utmost importance.
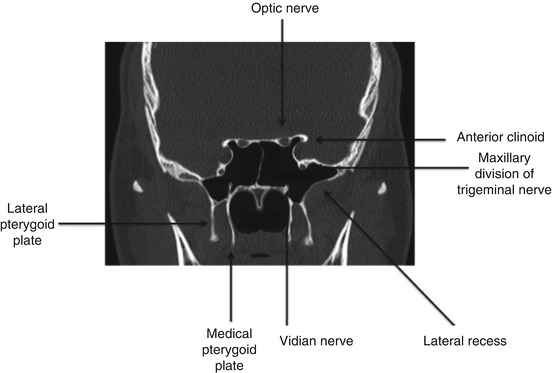
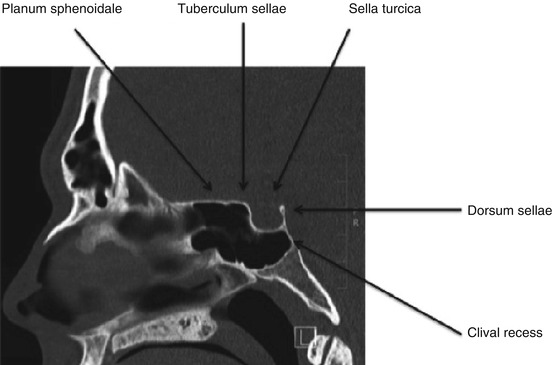

Fig. 27.2
Coronal computed tomography view of a well-pneumatized sphenoid sinus

Fig. 27.3
Sagittal computed tomography view of the sphenoid sinus
The presence and location of the intersphenoid sinus septum are also highly variable. In an evaluation of CT axial cuts of 296 patients, Hamid et al. found that no septum was present in 10.8 % of the patients and a single intersphenoid septum was present in 71.6 % of the cohort. The insertion of the septum was at the lowest point in the sellar floor, in a central point, in 66.9 %, and the intersphenoid septum pointed toward the carotid canal in 4.7 %. An accessory septum was also seen in 10.8 % of patients. These visualized accessory septa were attached to the sellar floor in 37.5 % of the cohort and extended into the carotid canal in 62.5 % of patients. Multiple intersphenoid septa were found in 6.8 % of patients. In this study, the intercarotid distance ranged from 12 to 30 mm with a mean of 23 mm [5].
Importantly, the degree of pneumatization within the sphenoid sinus can be significantly affected by chronic sinonasal disease. Frequent examples of this can be seen in patients with cystic fibrosis (CF) or primary ciliary dyskinesia (PCD) as these patient cohorts tend to have under-pneumatization with resultant hypoplastic sphenoid sinuses. While the definitive reason for this is unknown, one theory suggests that it is a result of thick secretions obstructing the sphenoid ostium, causing inhibition of aeration and subsequently stunting the normal development of the sinus [2]. It has also been shown that CF patients that are homozygous for the delta-F508 mutation have a greater incidence of sphenoid hypoplasia than CF patients with other genotypes [9].
In addition, it should be noted that arachnoid granulations often occur in close proximity of the maxillary division of the trigeminal nerve in the pterygoid recesses of well-aerated sphenoid sinuses [2, 10]. This anatomic finding has been strongly associated with spontaneous cerebrospinal fluid leaks or meningoencephaloceles within the lateral recesses of well-aerated sphenoid sinuses [10]. A recent evaluation of radiographic findings of 77 sphenoid lateral recess encephaloceles in 59 patients, identified arachnoid pits in 93 %, anterior cranial fossa skull base attenuation in 80 %, and empty sella in 75 % of the cohort. Previously it was believed that sphenoid lateral recess encephaloceles arose congenitally in “Sternberg’s canal,” a canal extending from the junction of the body of the sphenoid and lesser wing to the pharynx via a course medial to the superior orbital fissure. However, recent authors have refuted this claim as such a canal would lie medial to the foramen rotundum, but the vast majority of encephaloceles reported in the lateral sphenoid lie lateral to the foramen rotundum and therefore do not fulfill this critical criterion. The authors conclude that CSF leaks/encephaloceles at this location are likely the results of lateral pneumatization in the setting of an attenuated sphenoid sinus recess roof and development of arachnoid pits secondary to underlying intracranial hypertension [11].
Sphenoethmoidal cells, commonly referred to as Onodi cells, may also be present and should be identified preoperatively. These are posterior ethmoid cells that pneumatize posterior to the face of the sphenoid and may have direct communication with the optic nerve and/or carotid artery [10] (Fig. 27.4




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree






