Fig. 29.1
A patient with massive right frontal sinus posterior table erosion on coronal (a) and axial (b) CT scans with heterogeneous densities suggesting AFRS. The corresponding coronal (c) and axial (d) T2-weighted MRI scans show complete dropout of the signal in these areas
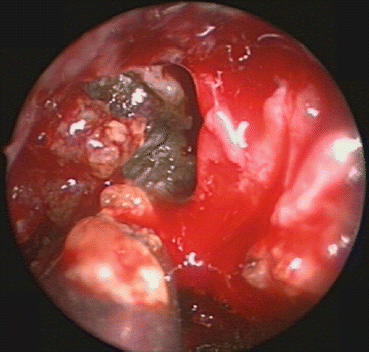
Fig. 29.2
Transnasal endoscopic view of allergic fungal mucin being removed from the frontal sinus. Note the peanut butter viscosity typical of AFRS
Epidemiology
AFRS is usually identified in patients who are young adults with a long history of nasal congestion, immunocompetence, unilateral or asymmetric involvement of the paranasal sinuses, nasal casts, hyposmia or anosmia, and facial pressure without significant pain. These findings cannot be used for diagnosis of AFRS, but they are usually present in people who have AFRS [6, 7]. Studies have estimated that anywhere from 5 to 10 % of patients who have been diagnosed with CRS are actually suffering from AFRS. AFRS is much more common in the southeastern and southwestern portion of the United States, including the Mississippi River basin. This geographical predominance is linked to the areas with a temperate climate and increased humidity, which is more conducive for the fungus to flourish [8]. In the southeastern portion of the country, up to one third of the people undergoing functional endoscopic sinus surgery are found to have fungal sinusitis [6]. AFRS is associated with a type 1 hypersensitivity reaction and has been likened to allergic bronchopulmonary aspergillosis (ABPA) in that they are both chronic inflammatory conditions utilizing the same facets of the immune response. Both of these disease processes are set in motion by an allergic response to small numbers of fungus present in the respiratory tract [4].
Pathophysiology
Increasing evidence over the years indicates that allergens and fungus play a large role in the pathogenesis of CRS. However, many questions regarding the cause of nasal polyposis remain unanswered. The development of CRS with nasal polyps may be associated with allergens – a claim bolstered by immunological evidence that Th2 (helper T cells) and eosinophils are the predominant cell types present. In contrast, acute rhinosinusitis due to bacterial causes or mucociliary dysfunction (e.g., cystic fibrosis) is associated with a neutrophilic response [9–13]. In some cases, it is thought that fungus incites an inflammatory response that is a separate trigger for CRS in the absence of a type 1 IgE hypersensitivity reaction [14]. Although the exact mechanism of a noninvasive allergic response in AFRS is still uncertain, the most well-accepted theory on the development of the disease is as follows: (1) a previously atopic patient is exposed to a fungus, (2) the fungus incites an antigenic inflammatory response via type 1 and type 3 hypersensitivity reactions in the nasal airway leading to eosinophilic accumulation and mucosal hypertrophy, (3) the fungus is able to propagate in a closed system when coupled with previously present mechanical or anatomical obstructions (occluded ostia or polyposis), (4) the propagation of the fungus leads to the eosinophilic mucin that continues to obstruct the drainage of the paranasal sinuses, and (5) further proliferation of the fungus stimulates a vicious inflammatory cycle [8, 15]. In contrast to that theory, Ponikau [16] suggested that fungus was present in nearly all nasal mucosa of CRS patients. Further studies of these patients noted that they did not have a fungal-specific antigenic or inflammatory response and that the response did not depend on IgE-mediated reactions but instead was secondary to T-lymphocyte-mediated inflammatory cascades that ultimately incite eosinophilic chemotaxis and activation. Regardless of controversies over fungal etiology, individuals with classical AFRS have type I hypersensitivity to molds that are usually very robust and will be the focus of the current chapter.
Medical Treatment
The recurrence rate of AFRS is considered high in patients with incomplete treatment. Therapy consists of both surgical (where fungal load should be removed) and postoperative medical interventions [8]. Medical therapy alone may not be enough for treatment of AFRS.
Oral Steroids
Like ABPA, oral steroid therapy is a mainstay of treatment for AFRS preoperatively due to the similarities in the pathophysiology of the two conditions. Corticosteroids limit the inflammatory reaction and cell recruitment to the mucosa by biochemically limiting IL-5, which is necessary for eosinophil chemotaxis and decrease vessel permeability [17]. In studies performed on AFRS patients, all patients eventually suffered a recurrence of AFRS if they were not treated with corticosteroids, and the time to surgical revision was extended for those that continued steroids for an extended period of time (1 year) [17, 18]. While systemic corticosteroids have been shown to be beneficial, risks are high with long-term and high-dosage therapy. These complications can include the following: growth retardation, diabetes mellitus, hypertension, psychotropic effects, gastrointestinal side effects, cataracts, glaucoma, osteoporosis, and aseptic necrosis of the femoral head [17]. Separating administration of systemic steroids to 3-month intervals can reduce the risks of the deleterious side effects but should be performed with informed decision from the patient. However, in a study by Schubert and Goetz [17], there were no complications reported with systemic corticosteroid treatment in the short term. Recommendations for preoperative and postoperative treatment with systemic steroids have been proposed. One of the accepted regimens for preoperative treatment is 0.5–1.0 mg/kg prednisone per day for 1 week before surgery to minimize inflammation and polyp volume [19]. It is also routinely accepted that steroids provided for greater than 2 weeks should be tapered over a period of time to prevent an Addisonian crisis.
Topical Steroids
Similar to systemic corticosteroids, topical corticosteroids limit the recruitment of inflammatory cells to the mucosa and reduce the edema and swelling that occurs in response to fungal antigen presentation. However, they have fewer complications than systemic steroids since the topical steroids have minimal systemic absorption. Unfortunately, topical steroid use is limited in the preoperative period because of poor penetration into the sinuses secondary to obstructive polyps and mucin. Postoperative administration is considered a critical therapy for AFRS because of the smaller side effect profile and limited systemic absorption when compared to oral steroids. Overall, the safety profile of the newer generation nasal corticosteroids is excellent, but there is still a risk of nasal bleeding and septal perforation. The risk of hypothalamic pituitary axis dysfunction can be seen with topical steroid when inhalational steroids are used in asthmatic patients [8]. When possible, patients should be provided nasal steroids with the smallest amount of systemic absorption (e.g., mometasone) for long-term administration. Because the allergy to molds will remain unless durable desensitization is performed, many individuals with AFRS may require prolonged treatment. Another option for acute exacerbations is the local application of steroid to the sinuses in an absorbable vehicle such as triamcinolone in carboxymethylcellulose foam [20].
Nasal Saline Irrigation
Nasal saline irrigation is a cheap and well-tolerated adjunct to other medical therapies for CRS with and without polyps, including AFRS. Randomized, controlled trials have found that sinus symptoms were reduced, and quality of life measures were improved with the use of saline irrigation. Compounded mometasone or budesonide mixed in saline rinse has become an attractive option for treatment because delivery to the sinus mucosa is greatly improved, while overall exposure to the drug may be less than a nasal steroid spray. This has become standard therapy in many institutions due to the belief that better treatment outcomes occur with more widespread sinus treatment [21].
Systemic and Topical Antifungal Therapy
Antifungal therapy was initially used for treatment because of the repeated findings that people without any conjugate treatment following surgery were very likely to relapse. Amphotericin B, with its high side effect profile, was exchanged for the drugs itraconazole and ketaconazole. Historical studies suggested antifungals used in the treatment of ABPA resulted in decreasing IgE levels and reduced need for corticosteroids [22]. This data was used as evidence to support this treatment option for individuals with AFRS [23]. However, the risk of drug-related morbidity and the cost of the drugs limit how useful they may be in clinical practice [22]. The effectiveness of antifungal therapy has also been questioned due to the lack of histological evidence of any tissue invasion [24]. Topical antifungal therapy with amphotericin B has also been suggested for treatment of AFRS due to a low toxicity profile. Theoretically, antifungal therapy could help prevent recurrence of AFRS by decreasing the fungal burden, which would reduce antigen exposure in an atopic individual [25]. A recent meta-analysis regarding antifungal therapies for CRS concluded that there was no support for treatment with this modality [26]. However, studies generally did not separate AFRS patients from other forms of CRS.
Immunotherapy
Initial concerns that immunotherapy would be harmful in the treatment of AFRS were unfounded. Studies conducted by Mabry [27, 28] found that there was no decline in patient outcomes or increase in patient symptoms within the first year of treatment with immunotherapy. Some patients were able to forego systemic corticosteroid therapy and eventually discontinue topical corticosteroid therapy. Furthermore, subjects who maintained the regimen had a smaller likelihood of recurrence up to 2 and 3 years of clinical follow-up [28]. Recurrence rates were also significantly lowered in patients who received surgical treatment and immunotherapy when compared to a surgical cohort alone [29].
Hypersensitivities to fungal and non-fungal antigens are usually treated in AFRS. Antigens are delivered separately, with fungal antigens in one vial and non-fungal antigens in a separate vial with separate spots of injection. This allows for monitoring of any allergic or delayed hypersensitivity reactions and the ability to discern which injection is causing the local reaction. Once maintenance level dosages have been reached, the two vials can be combined to decrease the number of injections and improve efficiency of the process [8]. Dosage adjustment follows the guidelines for other allergy shots, and the duration of treatment is typically 3–5 years [30].
Originally, it was suggested that immunotherapy be directed against the fungal antigens that were cultured out of the paranasal sinuses, but this overlooked a crucial aspect of fungal culturing. There is a noted inconsistency and unreliability to culture technique and fungal spore numbers on culture (e.g., high detection with PCR). This could lead to incomplete treatment [8, 28].
Risks of immunotherapy include increasing the patient inflammatory response if they continue to be exposed to a large antigen load while receiving immunotherapy injections [8]. Previous small-scale studies looking at the comparison of treatment with immunotherapy before and after surgery found that patients who received injections prior to surgery began to get worse with treatment or failed to improve, while those postsurgery had better outcomes [8]. If AFRS and ABPA concurrently exist in the same patient, immunotherapy can be risky because there is no good surgical intervention to remove ABPA (aside from bronchoscopy) [31].
Surgical Intervention
Because clinical and radiographic findings of AFRS may include “invasive” features such as orbital/skull base erosion or cranial neuropathies, destructive surgical procedures were often employed (Fig. 29.3). Osteoplastic flaps, cranializations, lateral rhinotomies, and facial degloving procedures were intended to eliminate the disease and the mucosa because of “invasion” into surrounding structures. Even with less aggressive intranasal procedures (e.g., sphenoethmoidectomy), the intent was to completely remove the mucosa [32]. These approaches carried high morbidity, symptoms would return, and patients required further surgery [33, 34]. For example, Sarti et al. describe a subject diagnosed with “paranasal aspergillosis” that was “invading the sella turcica and the anterior cranial fossa.” Despite no histological demonstration of invasion into the mucosa, a craniofacial resection was performed, and the patient subsequently died due to a thromboembolism [35].


Fig. 29.3
A patient presents with a left abducens palsy on leftward gaze secondary to compression from skull base erosion at the petrous apex
The gradual accumulation of allergic mucin in the sinus cavity causes the bony remodeling and decalcification that can resemble “invasion” on radiographic images. The areas of the bone that demineralize and the expansive areas of disease are directed by the inflammatory process in the area and the pressure of the accumulating mucin (Fig. 29.4) [35




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree






