Topical Corticosteroids: Introduction
|
Mechanism of Action
Corticosteroids have specific and nonspecific effects that are related to different mechanisms of action, including anti-inflammatory, immunosuppressive, antiproliferative, and vasoconstrictive effects. Most of their actions are mediated by an intracellular receptor called the glucocorticoid receptor. The glucocorticoid receptor α-isoform is located in the cytosol, binds glucocorticoids, and translocates to a region of the nuclear DNA known as the corticosteroid responsive element, where it is then able to stimulate or inhibit transcription of the adjacent genes, thus regulating the inflammatory process.1 The glucocorticoid receptor β-isoform does not bind glucocorticoids, but is able to bind the antiglucocorticoid/antiprogestin compound RU-486 to regulate gene expression.2 The glucocorticoid receptor β can attenuate the ligand-mediated transactivation of hormone-sensitive genes by the α-isoform and may be an important marker of steroid insensitivity.3
Corticosteroids are thought to exert their potent anti-inflammatory effects by inhibiting the release of phospholipase A2, an enzyme responsible for the formation of prostaglandins, leukotrienes, and other derivatives of the arachidonic acid pathway. Corticosteroids also inhibit transcription factors, such as activator protein 1 and nuclear factor κβ, which are involved in the activation of proinflammatory genes. Genes known to be upregulated by corticosteroids and that play a role in the resolution of inflammation include lipocortin and p11/calpactin-binding proteins, both involved in the release of arachidonic acid.1,4,5 Lipocortin I inhibits phospholipase A2, reducing the release of arachidonic acid from phospholipids.1,6,7 Corticosteroids also decrease the release of interleukin-1α (IL-1α), an important proinflammatory cytokine, from keratinocytes.1,8 Other proposed mechanisms for the anti-inflammatory effects of corticosteroids include inhibition of phagocytosis and stabilization of lysosomal membranes of phagocytizing cells.9
The effectiveness of corticosteroids is, in part, also due to their immunosuppressive properties. Corticosteroids suppress the production and effects of humoral factors involved in the inflammatory response, inhibit leukocyte migration to sites of inflammation, and interfere with the function of endothelial cells, granulocytes, mast cells, and fibroblasts.1,10–12 Several studies have shown that corticosteroids can cause mast cell depletion in the skin.13 Experiments have also shown that topical corticosteroids cause local inhibition of chemotaxis of neutrophils in vitro, and decrease the number of Ia+ Langerhans cells in vivo.14,15 Corticosteroids reduce eosinophilia in patients with asthma. They also reduce T-cell proliferation and induce T-cell apoptosis, in part from inhibition of the T-cell growth factor IL-2.1,16 In addition, several cytokines are directly affected by corticosteroids, including IL-1, tumor necrosis factor-α, granulocyte-macrophage colony-stimulating factor, and IL-8. These effects may also be a result of the steroid action on antigen presenting cells.17
The antiproliferative effect of topical corticosteroids is mediated by inhibition of DNA synthesis and mitosis, partly explaining the therapeutic action of these drugs in scaling dermatoses.18 They are known to reduce the keratinocyte size and proliferation. Fibroblast activity and collagen formation are also inhibited by topical corticosteroids.19
The mechanism by which corticosteroids induce vasoconstriction is not yet completely clear. It is thought to be related to inhibition of natural vasodilators such as histamine, bradykinins, and prostaglandins.1,20,21 Topical steroids cause capillaries in the superficial dermis to constrict, thus reducing erythema. The ability of a given corticosteroid agent to cause vasoconstriction usually correlates with its anti-inflammatory potency, and thus, vasoconstriction assays are often used to predict the clinical activity of an agent. These assays, in combination with double-blind clinical trials, have been used to separate the topical corticosteroids into seven classes based on potency. Class 1 includes the most potent, while class 7 contains the least potent. eTable 216-0.1 lists many of the available topical corticosteroids according to this classification. Notice that the same drug can be found in different potency classifications depending on the delivery vehicle used.
Generic Name Class 1—Superpotent
Class 2—Potent
Class 3—Potent, upper midstrength
Class 4—Midstrength
Class 5—Lower midstrength
Class 6—Mild strength
Class 7—Least potent
|
Pharmacokinetics
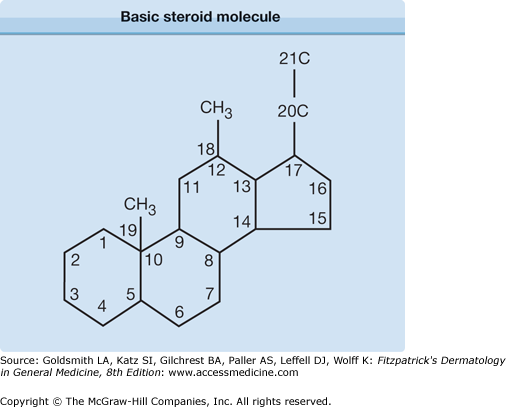
Corticosteroids have a basic skeletal structure comprising 17 carbon atoms arranged in three six-membered rings and one five-membered ring. Modifications of cortisol (Fig. 216-1), by addition or alteration of functional groups at certain positions, have led to compounds with variable anti-inflammatory potency, glucocorticosteroid versus mineralocorticoid activity, and adverse effects.22
Topical corticosteroid research has focused on strategies to optimize potency while minimizing side effects. One strategy is to develop compounds with enhanced anti-inflammatory effects and minimal unwanted atrophogenic and adrenal suppressive effects. In this sense, progress has been made with the development of glucocorticoid molecules that, while retaining high activity in the skin following topical application, are quickly broken down into inactive metabolites, thereby mitigating systemic and possibly some local toxic effects (“soft” glucocorticoids). Some of these compounds include the diesters 17,21-hydrocortisone aceponate and hydrocortisone 17-butyrate-21-propionate, prednicarbate, mometasone furoate, methylprednisolone aceponate, alclometasone dipropionate, and carbothioates such as fluticasone propionate.1,23 This last agent is classified as a potent corticosteroid with lower potential to cause skin atrophy and adrenal suppression due to its high lipophilicity, high glucocorticoid receptor binding and activation and rapid metabolism in the skin.24 It offers the advantage of once daily application and infrequent local allergic reactions. Mometasone furoate also has highly anti-inflammatory effects with low incidence of adrenal suppression.1 Hydrocortisone aceponate, prednicarbate, and methylprednisolone aceponate have significant anti-inflammatory effects, but the least capacity to induce skin atrophy; therefore, they can be used to treat areas such as the face, the scrotum, and large body surface areas in children, with minimal adverse effects.1,25
Before choosing a topical glucocorticoid preparation, one must consider the patient-related and drug-related factors that can affect its systemic absorption. The age of the patient, the extent and location of the body surface area to be treated and the presence or absence of skin inflammation, greatly affect the activity of the topical agent. Penetration of the glucocorticoid varies according to the skin site, which, in turn, is related to the thickness of the stratum corneum and the vascular supply to the area. For example, penetration of topical steroids through the eyelids and scrotum is four times greater than for the forehead and 36 times greater than for the palms and soles. Inflamed, moist, and denuded skin also shows increased penetration. Areas of the body where the skin is inherently thin not only allow for increased penetration of the drug but also are more susceptible to develop side effects than other areas where the skin is thick. Potent topical steroids (classes 1 and 2) should rarely, if ever, be used in the areas with the highest level of penetration, such as the eyelids. The concentration of the therapeutic agent used, the duration of the application, the use of occlusive dressings, the elected vehicle, and the intrinsic characteristics of the chosen molecule, can also affect the absorption and the degree of adverse effects.26,27 The target site for topical corticosteroids is the viable epidermis or dermis, and clinical response to a formulation is directly proportional to the concentration of corticosteroid achieved at the target site. A comparison study of skin concentrations after topical versus oral corticosteroid treatment found that most topical corticosteroids have the potential to achieve greater effective drug levels in the superficial layers of the skin than those achieved with standard doses of oral prednisone. Therefore, the apparently greater efficacy of oral corticosteroid therapy may be due in part to poor patient compliance with topical therapy.28
Topical corticosteroids are compounded in several formulations and with varying strengths. Recent research has emphasized the importance of treatment adherence in the management of skin conditions. As such, newer formulations including spray, foam, lotion, hydrogel, and shampoo formulations have been developed to improve patient convenience and acceptance, without sacrificing the efficacy, safety and tolerability of the traditional ointment and cream formulations. A recent systematic review of the literature found that while there are few direct comparison studies between clobetasol propionate, a class 1 steroid, in different vehicles, the efficacy rates for more recent formulations is roughly comparable to that of clobetasol ointment in the treatment of psoriasis. The most common adverse effect was mild and transient stinging/burning at the lesion site, which may be due to the alcohol content found in these formulations.29 None of the clinical trials directly compared these formulations with one another.30,31
Increasing hydration of the stratum corneum can enhance absorption of topical corticosteroids by four to five times. Absorption is also enhanced by ten times with occlusion.32 A retrospective study of wet dressings used with topical corticosteroids (hydrocortisone 1% cream to the face and folds and triamcinolone 0.1% cream from the neck down) for adults with recalcitrant pruritic dermatoses of different etiologies, alleviated the pruritus in 98% of the patients at dismissal. The enhanced corticosteroid penetration is only one of the numerous benefits of the wet dressings.33
Indications
Topical corticosteroids are recommended for their anti-inflammatory activity in inflammatory skin diseases, but they can also be used for their antimitotic effects and their capacity to decrease the synthesis of connective tissue molecules.1 Certain variables must be considered when treating skin disorders with topical glucocorticoids. For example, the responsiveness of diseases to topical glucocorticoids varies. In this setting, diseases can be divided into the three categories shown in Table 216-1: (1) highly responsive, (2) moderately responsive, and (3) least responsive.
Highly Responsive | Moderately Responsive | Least Responsive |
|---|---|---|
|
|
|
Topical glucocorticoids are highly effective, and few side effects are observed when a low-potency preparation is used for brief periods of time without occlusion in children. However, children and, in particular, infants, are at an increased risk of absorbing topical corticosteroids for several reasons. They have a higher ratio of skin surface area to body weight and application to a given area results in a greater potentially systemic dose of steroid. Infants may also be less able to metabolize potent glucocorticoids rapidly.34 Premature infants are especially at risk because their skin is thinner and the penetration rate of topically applied drugs is greatly increased.35 Application of topical steroids to the diaper area results in occlusion of the steroid by the diaper, and increased penetration occurs. Excess absorption of topical glucocorticoids can suppress endogenous cortisol production. Consequently, subsequent cessation of topical steroid therapy after an extended treatment period can, albeit rarely, result in an addisonian crisis. Deaths from addisonian crisis have been reported with the use of topical steroids, and the risk of this occurring is greater in children.36 Chronic suppression of cortisol production can also lead to growth retardation. A morning plasma cortisol level can be performed to screen for adrenal suppression, although ACTH stimulation testing with cosyntropin is more accurate. If suppression is present, the child should be slowly weaned from the steroids to prevent these complications.
Corticosteroids have been used with success for atopic dermatitis for several decades. Placebo-controlled trials have found them effective in 75% or more of patients with atopic dermatitis when compared with fewer than 30% of placebo-treated patients.37 They are important for managing acute flares. As with other skin conditions, selecting the appropriate strength according to the body site, the extent of involvement and the flare intensity is essential for treatment success.27 Education of the patients and caregivers is critical to improve adherence to the prescribed medication and optimize compliance. Results from large-scale surveys show that patients/caregivers overestimate the actual risks of topical corticosteroids (“steroid phobias”) leading to treatment noncompliance.38,39 Adequate time should be spent transmitting the important role of intermittent topical corticosteroid therapy, and the beneficial risk-benefit ratio with their appropriate use.40
Hemangiomas of infancy show a good or partial response to treatment with ultrapotent topical glucocorticoids in 74% of infants. The majority reported accelerated cessation of growth. Small, superficial hemangiomas, particularly at sites prone to ulceration, disfigurement or both, and small periocular lesions that have not yet caused significant visual impairment are the best candidates for therapy.41 The mechanism of action by which corticosteroids act in hemangiomas to decrease proliferation is unknown. Intralesional corticosteroid injection of hemangiomas before and after treatment, have revealed an increase in mast cells, reduced transcription in several cytokines and enhanced transcription of cytochrome b gene.42
Elderly patients similarly can have thin skin, which allows for increased penetration of topical glucocorticoids. They are also more likely to have preexisting skin atrophy secondary to aging and may be diaper dependent, so the same precautions used in the treatment of infants should be used when treating elderly patients.
Appropriate human studies using topical glucocorticoids in pregnancy have never been undertaken. Studies in animals, however, show that topical steroids are systemically absorbed and may cause fetal abnormalities, especially when used in excessive amounts, under occlusive dressings, for prolonged periods of time, or when the more potent agents are used. Most topical steroids are rated by the US Food and Drug Administration as category C drugs, which imply that caution must be exercised when used in pregnancy. A recent, systematic review of the safety of topical corticosteroids in pregnancy performed by Chi et al, found that the current data is inconclusive and limited and unable to detect an association between topical corticosteroids and congenital abnormalities, preterm delivery, mode of delivery or stillbirth. The current evidence shows no statistically significant effects for pregnant women who use topical corticosteroids compared with unexposed women. However, in a small cohort study of participants from a single maternity center, there appears to be an association of highly potent corticosteroids with low birth weight. Most of the previous studies only assessed the risk for congenital abnormality or orofacial cleft. Further cohort studies with comprehensive outcome measures (including fetal growth, preterm birth, and birth death), consideration of corticosteroid potency, dosage and indications, and a large sample size are required in order to detect a small risk.43 It is currently unknown whether topical glucocorticoids are excreted in breast milk; however, they should be used with caution in breastfeeding mothers and should never be used on the breasts before breastfeeding.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree