Authors
Years
Number of patients
Five-year survival (%)
Country
Miyauchi et al. [12]
1992
23
48
Japan
Dahlstrøm et al. [20]
1993
98
56
Denmark
Mora et al. [21]
1996
69
72
USA
Faneyte et al. [8]
1997
44
45
Netherlands
Downey et al. [3]
2000
38
18
USA
Henderson et al. [22]
2001
61
24
Australia
Moran et al. [23]
2002
53
55
USA
Friedel et al. [17]
2005
51
41
Germany
Veronesi et al. [4]
2007
15
19
Italy
Friedel et al. [2]
2008
63
46
Germany
Santillan et al. [5]
2008
28
18
USA
In a correctly selected group of patients undergoing chest wall resection after local recurrence of breast cancer, the primary goal is to regain local control regardless of the extent of disease. Some of these patients, in fact, will present with painful, infected, ulcerated, or fungating lesions that cause a great distress to the patients [5]. Treatment with radiotherapy, systemic therapy, and surgery, alone or in combination, can help to achieve local control [15, 16].
The reported overall operative mortality rate after chest wall resection for breast cancer recurrence is fairly low, ranging from 0 % in most of the recent series [3–5] up to 2.0 % [17]; however, a higher mortality rate, ranging form 3.5 to 4.5 %, has been reported [9], and a 30-day mortality rate of 7 % has occasionally been reported [18]. In contrast, the postoperative complication rate is not negligible. Minor postoperative morbidity includes edge necrosis of the myocutaneous flap requiring surgical excision with the patient under local or general anesthesia, and pleural effusion requiring pleural drainage. Reported major complications are prosthesis infection, chest wall hematoma (requiring a re-do operation), massive atelectasis (requiring toilette bronchoscopy), and postoperative empyema. Morbidity has been reported to be 20–50 % in various studies, with a rate of reintervention ranging from 17 to 22 % [2].
On the basis of the existing literature, we may argue that complete resection with free margins is recommended as the first choice for treatment in recurrent breast cancer. In fact, local recurrence has to be regraded as a repeated episode of a disease with an increased risk of subsequent metastases and not vice versa. The curve of metastatic incidence might be flattened or reduced markedly by a radical resection with sufficient safety margins [2].
Risk factors affecting long-term survival are a diameter of the local recurrence greater than 1.5 cm, disease-free interval of less than 2 years, skin incision, initial tumor stage, and positive lymph nodes [19]. Age at the time of primary resection has been described as a prognostic factor, although different cutoff and prognostic values have been reported. Faneyte et al. [8] observed that patients who were younger than 35 years at the time of primary therapy had significantly lower survival rates after the resection of a chest wall recurrence. In contrast, Friedel et al. [2] observed that the distinction between younger and older patients was determined to be 45 years, with an improved long-term survival for the younger group, who had better prognosis with surgical therapy for the local recurrence than older patients. Whether this is actually due to the therapeutic procedure or the generally decreased life expectancy of older patients has not been clarified yet.
44.3 Technical Aspects
The previously implanted breast prosthesis is removed (Figs. 44.1, 44.2, 44.3). The chest wall is exposed and every involved rib is prepared with a periosteal elevator (Figs. 44.4, 44.5). The intercostal vein and artery are dissected and ligated to allow a safe rib resection with a rib cutter (Figs. 44.6, 44.7, 44.8). The mammary vein and artery of the involved hemisternum are dissected, isolated, and then ligated (Figs. 44.9, 44.10). The sternum is then transected, both horizontally and vertically, with a sternal saw (Figs. 44.11, 44.12). The chest wall resection is then completed, exposing intrathoracic structures. Interrupted multiple nonabsorbable stitches are roundly placed for subsequent fixing of the prosthesis (Figs. 44.13, 44.14). A polypropylene prosthesis is prepared with resinous material according to the extent of parietal defect following chest wall resection (Fig. 44.15). The prosthesis is then implanted and fixed to adjacent healthy bones of the chest wall (Fig. 44.16). The latissimus dorsi muscle flap is then prepared, with a cutaneous island, and then rotated to cover the prosthesis and to close the anterior tissue defect (Figs. 44.17, 44.18).
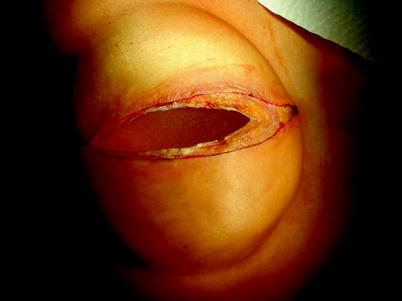





Fig. 44.1
The previously implanted breast prosthesis is removed
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








