28 Therapeutic radiation
Principles, effects, and complications
Synopsis
 Radiation therapy (RT) can be used as a primary or adjuvant treatment for many cancers.
Radiation therapy (RT) can be used as a primary or adjuvant treatment for many cancers.
 The unit of dose measurement is the gray (Gy).
The unit of dose measurement is the gray (Gy).
 Side-effects are characterized as acute and late toxicities.
Side-effects are characterized as acute and late toxicities.
 Acute toxicities are temporary and reversible.
Acute toxicities are temporary and reversible.
 Late toxicities are permanent.
Late toxicities are permanent.
 The primary mechanism of action of radiation is through breakage of DNA strands.
The primary mechanism of action of radiation is through breakage of DNA strands.
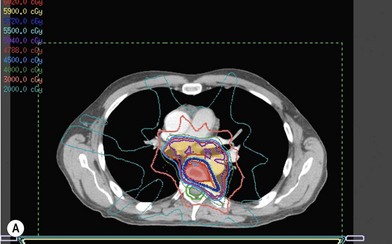
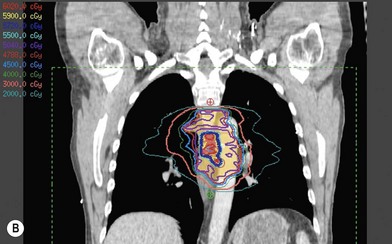
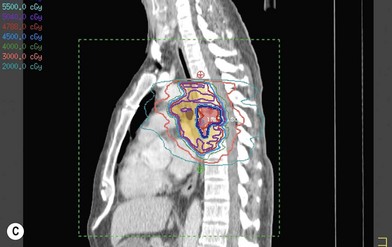
 Treatment planning is achieved through use of three-dimensional computed tomography (CT) planning.
Treatment planning is achieved through use of three-dimensional computed tomography (CT) planning.
 Delivery of radiation can be very precise with the use of intensity-modulated radiation therapy (IMRT) as well as with image-guided radiation therapy (IGRT).
Delivery of radiation can be very precise with the use of intensity-modulated radiation therapy (IMRT) as well as with image-guided radiation therapy (IGRT).
Historical perspective
In November 1895, Wilhelm Conrad von Roentgen discovered a new type of ray that was generated from a stream of electrons in a cathode gas discharge tube and had the ability to pass though tissues with different degrees of penetration. Because the composition of the rays was unknown, they were called X-rays. His subsequent presentation to the Wurzburg Society on the ability of his rays to generate an image of structures inside the body gave birth to diagnostic radiology. The following year, in France, Henri Becquerel discovered that fluorescent materials made of uranium also emitted constant radiation.1 Marie and Pierre Curie used ionization to measure becquerel rays and quantify radiation intensity against uranium. Marie Curie first used the word “radioactivity” in 1898. The couple isolated two new radioactive elements from pitchblende, polonium, named for Marie’s native Poland, and radium, Latin for ray. Today, these pioneers’ names are in constant use as measures of radioactivity.2
The earliest oncologic brachytherapy procedure was in 1902, when a radium applicator was used to treat a tumor of the palate and pharynx. Within a few years, there were clinical studies of effectiveness, and radium tubes were inserted directly into tumors, and by the 1920s, radium had become the standard of care in oncology,3 as well as the treatment for many benign conditions. The use of Roentgen’s rays to palliate advanced cancer was first described in 1903,4 and this was the forerunner of external-beam radiation treatment.
Introduction
RT is the use of ionizing radiation to treat malignant tumors and some benign disorders. Nearly two-thirds of cancer patients receive RT as part or all of their treatment. Radiation oncology is the discipline of medicine that addresses the causes, prevention, and treatment of human cancer with special emphasis on the role of ionizing radiation.4 Radiation oncologists work in a multiprofessional context with radiation therapists, nursing, dosimetry and medical physics, along with dieticians, social workers, and counselors. Radiation therapists are the technologists who deliver the RT. Unlike their counterparts in diagnostic radiology, they spend not only considerable time on the technical components of treatment (treatment delivery, quality assurance, and verification), but also interact with and support cancer patients throughout the duration of their therapy. The radiation oncologist is also part of a multidisciplinary team, with specialized surgical and medical oncologists, along with diagnostic radiology and pathologists, which advocates for the best evidence-based care for their cancer patients. However, outside the immediate team, it is rare for other physicians to know much about the process, rationale, and issues around RT, and even less about the risks and causes of toxicity, let alone the technology involved. This chapter is designed as a primer, or virtual elective in the topic, and aims to help improve communication and understanding between plastic surgeons and radiation oncologists so that they can better appreciate some of the common issues that they have to deal with. This chapter starts with a brief description of the basics of radiation technology, modalities, medical physics, and radiobiology. It then outlines practical applications, with a description of RT planning and process, and clinical treatment issues. The final section describes specific toxicity syndromes.
Radiation technology
Megavoltage radiotherapy allows even greater penetration of tissue, resulting in better skin-sparing and improved dose homogeneity. Cobalt units deliver MV radiation, and have a 60Co source that generates gamma (γ) rays with average megavoltage energy of 1.3 MV. 60Co has a half-life of 5.26 years, and, because of this decay and subsequent drop in output, the source needs to be replaced every few years. Maximum dose (Dmax) is not achieved until 0.5 cm beyond the surface of the skin and the intrinsic scatter of the beam and ionized particles means that the penumbra, or dose fall-off at the edge of a beam, is not very tight. Despite this, cobalt is still the most prevalent and important external-beam radiotherapy modality in the world. Although affluent countries have mostly replaced their cobalt units with linear accelerators, cobalt still provides an excellent low-maintenance, reliable, safe, and effective alternative in many low- and middle-income countries.
Particle therapy
Neutron RT offers the advantage of high linear energy transfer, a property that allows the delivery of 20–100 times more energy along their path than photons, thus being biologically more effective in treating radioresistant tumors such as sarcomas and salivary gland tumors. Their use requires a careful balance of risk and benefit as they can lead to more severe long-term side-effects, due to relatively high effective entrance and exit doses.5
Physics
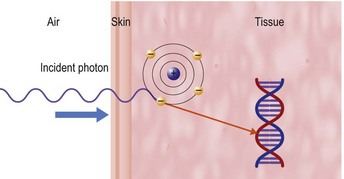
An explanation of RT techniques and dosimetry requires a basic understanding of physics. The photon generated by the linac is a very high-energy X-ray that does not have any charged particles in it. However, when it enters the body, it passes through tissue faster than the speed of light. As it goes through the skin and enters the tissue, it uses the Compton process to deposit energy. This means that it interacts with biological tissue by colliding with an electron on the more loosely bound outer shell of an atom (Fig. 28.1). The photon continues along its path, but the electron is knocked off, like a billiard ball, at an angle that depends on the speed and angle with which it was hit. The electron travels (scatters) a distance that is proportional to the energy with which it was hit, meaning that higher photon energies propel electrons more in a forward direction than lower energies can do, before the electron is deposited, and the dose absorbed into the tissues. This triggers downstream free radical interactions, and these can cause DNA single-strand or double-strand breaks, or other injuries, including damage to basepairs and protein cross-links. The chromosomal aberrations that result from double-strand breaks mean that the cancerous cell cannot repair or duplicate at the end of its life cycle, and this is the principal cause of cell death.
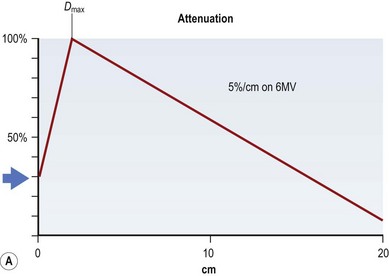
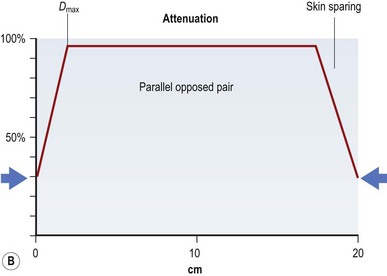
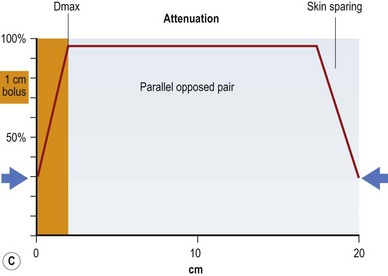
If all of these electron energy deposits are added up, then a distribution curve showing absorbed dose and depth can be plotted (Fig. 28.2A) that shows that skin absorbed dose is less than 40%, and 100% or Dmax occurs some distance into the skin at a depth that is consistent with the energy of the photon beam. The commonest energy used is 6 MV, and this has a Dmax of 1.5 cm beyond skin. The dose then falls off at a steady rate that is just less than 5%/cm, meaning that 50% of the dose has been deposited approximately 11 cm into the body. If a tumor is situated centrally, this means that there is too much of a gradient of dose across it. To overcome this heterogeneity within the target volume, a second beam can enter the body (assuming a diameter of 20 cm) from the opposite side, again depositing its maximal energy at 1.5 cm, then attenuating at <5%/cm. The energy deposits from each side are added, a homogeneous distribution is achieved across the target volume (Fig. 28.2B), and the skin does not receive a therapeutic dose, unless bolus is used (Fig. 28.2C). This explains the technique of parallel-opposed pairs (of beams). Similarly, assuming the example, above used opposing anterior and posterior beams, then the addition of right and left lateral beams (four-field box technique) would give an even tighter homogeneous distribution to the target volume, and, since the entrance and exit doses are shared between four beams, further skin dose reduction. This is the basis for using multiple fields, or beams, from different angles, to “focus” (a misleading term, since the beam does not focus on a spot, as light does through a lens) and deposit maximal dose to a target.
In conventional RT, most beams are delivered with uniform intensity across the field. Beam-modifying devices are accessories such as shielding blocks, wedges, and compensators, which are placed in the head of the machine to differentially absorb dose proportionally to the thickness of the device (i.e., more dose is absorbed through thicker regions, and less through thin), and thus reduce hot or cold spots in tissues that have nonsquare shapes. In modern linacs, blocking is done by a system of multileaf collimators (MLCs), which are narrow strips of metal in the head of the linac that act like miniblocks. Their shape can be programmed for shielding (Fig. 28.3). Three-dimensional conformal therapy uses multiple beams to be shaped in such a way that a significant amount of normal tissue can be excluded.
MLCs can also be programmed to move dynamically in and out of the beam, so that the amount of time in each position is adjusted to allow a variable amount of dose through each leaf, compensates for irregular shapes with great precision, and can control the dose to specified targets within a volume. This is the basis for IMRT, which involves identifying doses for individual or multiple target volumes and constraints for normal organs that need to be protected from the effect of radiation, and then using a sophisticated RT planning program that modulates the intensity across multiple individual beams or arcs with dynamic MLC, using an inverse planning system. This allows the creation of a very conformal plan that can even have convex and concave shapes to avoid a critical structure, for example, avoiding the spinal cord in a way that conventional beams would not be able to do (Fig. 28.4). It is also possible to “dose paint” so that different areas receive different doses at the same time, and also to reduce volumes, adapting to reduction in the tumor size. A similar result is obtained with tomotherapy, which uses principles of CT scanning to deliver intensity-modulated beams slice by slice. An advantage of IMRT over this system is that IMRT is delivered on a normal linac rather than a dedicated machine.
Radiobiology
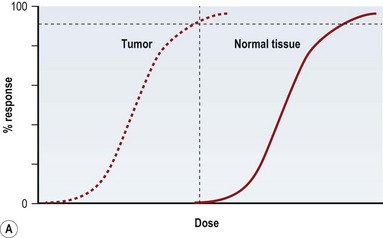
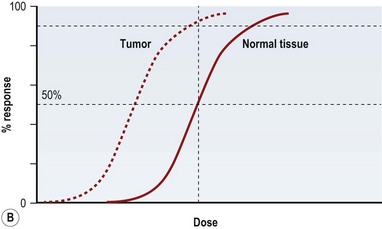
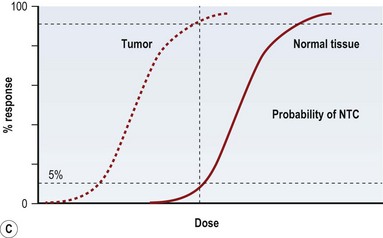
Radiation injury can be viewed from two perspectives: the radiation effect on tumor cells, and the radiation toxicity on normal cells. The therapeutic ratio is a dose–response relationship between tumor control and normal tissue complications, and helps balance the two perspectives. In an ideal world, the curves representing tumor response and normal tissue complications would be well separated (Fig. 28.5A). Far too often, they are so close together that it is not possible to achieve a high enough dose to eradicate the tumor without causing harmful complications (Fig. 28.5B). Usually in clinical practice there is some degree of compromise, and traditionally, a 5% risk of normal tissue complications is accepted (Fig. 28.5C), taking into account the specific risks of the situation.
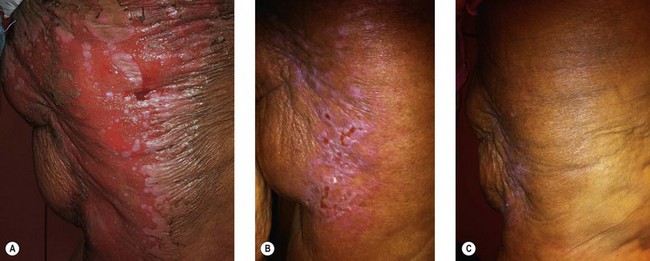
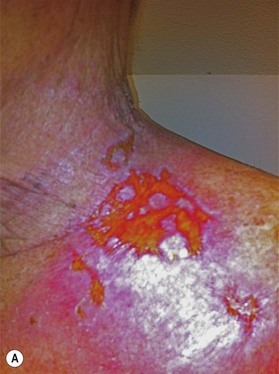
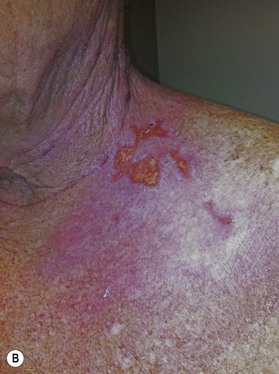
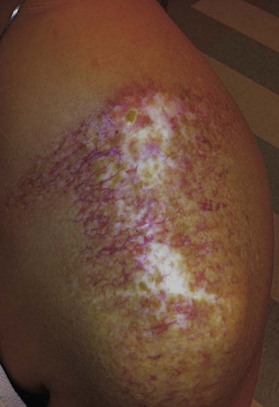
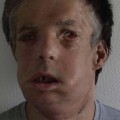
Radiation toxicity is expressed during mitosis of normal cells. Acute toxicity occurs during treatment or shortly afterwards. It is related to the total dose, the size of the volume irradiated, and radiosensitizing drugs such as chemotherapy. It occurs in cells with a rapid turnover, such as oral mucosa or skin epithelium, where cell division is necessary to maintain function. Even though these cells may be affected within a few days of starting fractionated treatment, their damage does not become manifest for at least 2 weeks as the progenitor stem cells do not have as rapid a turnover. Examples are skin erythema and desquamation, oral mucositis, and esophagitis. These reactions usually start to heal 2 weeks after the completion of treatment. Even quite brisk reactions can heal fast (Figs 28.6, 28.7). Acute toxicity is reversible, and does not predict for permanent damage. The exception is if there is consequential damage, i.e., there is persistent injury due to the destruction of the basement membrane zone with complete depletion of stem cells due to very high dose, and usually an additional factor such as chemotherapy, infection, or trauma. This can be found in chronic ulcers in the skin, bladder, or gastrointestinal tract. Delayed acute toxicity occurs 6 weeks to 6 months after the completion of treatment; it is also reversible. As with acute toxicity, it is dose-, volume-, and drug-related. Examples are radiation pneumonitis and L’Hermitte’s syndrome (a transient demyelination syndrome of the spinal cord, which does not predict subsequent radiation myelitis).
A method of quantifying the risk of permanent tissue damage is the concept of TD5/5, which is the total dose, given in standard fraction sizes, that produces a 5% risk of damage to a specified organ at 5 years.6 These figures have been updated to reflect modern conformal treatments7 and provide a guide for constraining the dose to an organ at risk during the treatment-planning process, as well as for guiding informed consent.
Response to radiation injury causes a signal transduction pathway that is mediated by the ataxia telangectasia mutated (ATM) gene to instigate DNA repair and to regulate cell cycle checkpoints, giving the cells additional time to repair. A cascade of cytokines is an immediate response, and may lead to both radiosensitization and protection.8 Interleukin-1 and 6, and tumor necrosis factor-α cause an inflammatory response and coagulation effect. Transforming growth factor-β (TGF-β) is one of the most widely studied radiation-induced cytokines, and plays multiple roles. It causes fibroblast differentiation as well as stimulating the production of collagen. It is involved in the synthesis and regulation of extracellular matrix molecules. Following radiation, the extracellular matrix is both quantitatively and qualitatively modified – degrading progresses are altered and production of collagen synthesis increases. TGF-β also down-regulates thrombomodulin activity in endothelium, leading to platelet aggregation, and, from the platelets, release of more TGF-β, thus setting up a self-perpetuation production of TGF-β that contributes to the chronicity of radiation injury. However, it is primarily the sustained activity of TGF-β-driven myofibroblasts that causes the fibrotic reactions to radiation. Fibrosis is not an endpont, but a dynamic process of fibroblast activity. In traumatic wounds, remodeling happens over years, but this capacity to remodel in lost in irradiated tissue and instead there is self-perpetuating reactive fibrosis.9
Irradiated skin and soft tissue are susceptible to minimal trauma, creating a problem for surgical intervention. Radiation causes delayed wound healing, with decreased wound-breaking strength because of the damage to the microvasculature and the decreased cellular elements.9 These problems are related to the total dose, the dose per fraction, the timing of the surgery, and the extent of the surgery. The optimal time to operate on irradiated skin is the window provided once the acute reaction has resolved and before the development of excessive fibrosis, generally 3–10 weeks after completion of XRT (Fig. 28.8).