3 Botulinum toxin (BoNT-A)
Synopsis
 When properly administered to appropriately selected individuals, botulinum toxin type-A (BoNT-A) can have a rejuvenating effect on nearly the entire face and neck.
When properly administered to appropriately selected individuals, botulinum toxin type-A (BoNT-A) can have a rejuvenating effect on nearly the entire face and neck.
 Carefully observing the functional anatomy of each patient is the key to determining how much BoNT-A to administer and where to place each injection.
Carefully observing the functional anatomy of each patient is the key to determining how much BoNT-A to administer and where to place each injection.
 In some facial areas, fine control can be achieved by only partially affecting muscles, causing the neighboring musculature to compensate for the ensuing weakness.
In some facial areas, fine control can be achieved by only partially affecting muscles, causing the neighboring musculature to compensate for the ensuing weakness.
Introduction
BoNT-A treatment is currently the most frequently performed cosmetic procedure in the US and its importance in aesthetic plastic surgery simply cannot be overstated. In 2008, nearly 2.5 million BoNT-A procedures were performed in the US, representing approximately 30% of all nonsurgical cosmetic procedures. That is more than all liposuction, breast augmentation, rhinoplasty, facelift, and blepharoplasty procedures combined.1
Key points
• The most important elements for facial rejuvenation with BoNT-A are functional anatomy, functional anatomy, and functional anatomy!
• To minimize the risk of a frozen, unnatural appearance, use the minimally effective dose of BoNT-A.
• Brow elevation depends on the relative weakness of the brow elevators versus brow depressors.
• The dose of BoNT-A used should be based on the estimated mass of the muscle being injected, not the depth of the rhytid.
• Avoiding the use of aspirin and non-steroidal antiinflammatory medications will decrease the occurrence and severity of ecchymosis.
• The lower frontalis muscle has the greatest effect on brow elevation.
• Threading the injection through the lips gives a more natural result than the near universal point technique.
• Cooling the skin before injection minimizes discomfort.
• As a general rule, injecting depressors more strongly than elevators will tend to give a more gentle lift to the area involved.
• Over-injecting the mentalis can result in a “witch’s chin” deformity and oral incompetence.
History
BoNT-A was first used in ophthalmology for the treatment of strabismus:
• Injecting BoNT-A into spastic extraocular muscles provided a noninvasive alternative to surgery.
• Effectiveness was first established in Rhesus monkeys.2
• The safety and effectiveness were later demonstrated in human patients.3
The ophthalmic use of BoNT-A soon included treatment of incapacitating essential blepharospasm:
The use of BoNT-A rapidly expanded into the field of aesthetic medicine:
Basic science
Pharmacology and pharmacokinetics
Clostridium botulinum is a Gram-positive, anaerobic bacterium that is known to produce seven serologically distinct types of toxin designated A through G, of which type A is the most potent. Botulinum toxin types A and B are used medically and are available in the US. The type-A toxin is a fully sequenced, 1296 amino acid polypeptide protein consisting of a 100-kDa heavy chain joined by a disulfide bond to a 50-kDa light chain.6
In the normally-functioning neuromuscular junction, the propagation of an action potential at the presynaptic neuron terminal opens voltage-dependent calcium channels. The influx of extracellular calcium ions causes vesicles containing acetylcholine to dock and fuse to the presynaptic neuron’s cell membrane through the action of a 25 kDa soluble N-ethylmaleimide-sensitive factor attachment protein (SNAP-25). The released acetylcholine crosses the synaptic cleft, where it binds with nicotinic receptors at the motor end plate, opens sodium-potassium ion channels, depolarizes the motor endplate, and initiates the sequence of events that leads to contraction of the muscle fiber.7
Following the administration of botulinum toxin, the heavy chain binds to the axon terminal, which enables the toxin to enter the neuron via endocytosis. In the cytoplasm, the proteolytic light chain degrades SNAP-25, thereby preventing fusion of the acetylcholine containing vesicle with the cell membrane, preventing release of acetylcholine. Within a few days, the affected nerve is incapable of releasing acetylcholine, resulting in flaccid paralysis of the muscle fiber it innervates. Type-B botulinum toxin also causes flaccid paralysis but does so by inhibiting synaptobrevin, a vesicle-associated membrane protein similar to SNAP-25.7 Unless specified, the remainder of this chapter concerns botulinum toxin type A.
Commercial sources of BoNT-A
Several botulinum toxin products are currently available in the US:
• Botox® (onabotulinumtoxinA) for injection (Allergan, Inc., Irvine, CA) is supplied in vials containing 100 U of vacuum-dried Clostridium botulinum type A neurotoxin complex, 0.5 mg of albumin human, and 0.9 mg of sodium chloride without a preservative.8
• Botox® Cosmetic (onabotulinumtoxinA) for injection (Allergan, Inc., Irvine, CA) is provided in vials containing 50 U of vacuum-dried Clostridium botulinum type A neurotoxin complex, 0.25 mg of albumin human, and 0.45 mg of sodium chloride without a preservative, or 100 U of vacuum-dried Clostridium botulinum type A neurotoxin complex, 0.5 mg of albumin human, and 0.9 mg of sodium chloride without a preservative.9
• Dysport™ for injection (abobotulinumtoxinA) (Tercica Inc., Brisbane, CA and Medicis Aesthetics Inc., Scottsdale, AZ) is supplied in vials containing 500 or 300 U of lyophilized abobotulinumtoxinA, 125 µg human serum albumin and 2.5 mg lactose.10
• Xeomin for injection (incobotulinumtoxinA) (Merz) in 50 and 100 unit vials.
Commercial source of BoNT-B
• Myobloc® (rimabotulinumtoxinB) injection (Solstice Neurosciences Inc., South San Francisco, CA) is provided in 3.5 mL vials containing 5000 U of botulinum toxin type B per mL in 0.05% human serum albumin, 0.01 M sodium succinate, and 0.1 M sodium chloride.11
The BoNT-A in Dysport and Botox are produced by fermentation of the bacterium Clostridium botulinum type A (Hall Strain), while BoNT-B in Myobloc is produced by fermentation of the bacterium Clostridium botulinum type B (Bean strain).12 It is important to note that the potency of each product is specific to the preparation and assay method utilized and is not interchangeable with other preparations of botulinum toxin products.13,14
Indications
Botox is indicated for the treatment of cervical dystonia in adults to decrease the severity of abnormal head position and neck pain associated with cervical dystonia and also for the treatment of strabismus and blepharospasm associated with dystonia, including benign essential blepharospasm or VII nerve disorders in patients ≥12 years of age. Botox is also approved for the treatment of severe primary axillary hyperhidrosis that is inadequately managed with topical agents.8 BoNT-A preparations are effective treatments for hyperhidrosis by decreasing cholinergic stimulation of eccrine glands responsible for sweat production.
Botox Cosmetic is indicated for the temporary improvement in the appearance of moderate to severe glabellar lines associated with corrugator and/or procerus muscle activity in adult patients ≤65 years of age.9
Dysport is indicated for the treatment of adults with cervical dystonia to reduce the severity of abnormal head position and neck pain in both toxin-naive and previously treated patients and also for the temporary improvement in the appearance of moderate to severe glabellar lines associated with procerus and corrugator muscle activity in adult patients <65 years of age.10
Myobloc (BoNT-B) is indicated for the treatment of adults with cervical dystonia to reduce the severity of abnormal head position and neck pain associated with cervical dystonia.11
The use of BoNT-A and BoNT-B for neurological purposes has become extensive. Its approved use for the treatment of cervical dystonia15 has grown to include limb spasticity and dystonias, hypersecretory syndromes such as sialorrhea, headache, low back pain16 and writer’s cramp.17
Warnings and contraindications
The use of products containing botulinum toxin is contraindicated in the presence of infection at the proposed injection site and in individuals with known hypersensitivity to any botulinum toxin preparation or to any of the components in the formulation. The use of BoNT-A is contraindicated in patients with disorders of neuromuscular transmission, such as myasthenia gravis or Lambert–Eaton myasthenic syndrome. Dysport contains a small amount of lactose and may contain trace amounts of cow’s milk protein. Dysport should not be use in patients with a known allergy to cow’s milk protein.10
Adverse effects
In the United States, due to reports received by the Food and Drug Administration regarding serious systemic adverse reactions including respiratory compromise and death following the use of botulinum toxins types A and B for both approved and unapproved uses,18 the labeling of all botulinum toxin-containing products are required to containing the following boxed warning:
Adverse events reported by the manufacturers of BoNT-A and BoNT-B products include nasopharyngitis, headache, injection site pain, bleeding, bruising, edema, erythema, infection, inflammation, sinusitis, ecchymosis and nausea.8–11 Weakness of adjacent muscles may also occur due to spread of toxin resulting in undesired effects. These types of adverse events are described in greater detail below. To minimize bruising, patients should avoid the use of aspirin and non-steroidal antiinflammatory medications for 2 weeks prior to treatment.
Patients have rarely developed neutralizing antibodies for BoNT-A following cosmetic use. These events are more commonly associated with the use of high doses for neurologic purposes19,20 although this has been reported following cosmetic use resulting in treatment failure.21
Dosing
These principles were demonstrated during a recent clinical study. Patients were stratified by demographics and randomized to receive a single treatment with different doses of BoNT-A (Dysport) or placebo.22 Based on procerus/corrugator muscle mass, women received doses of 50, 60, or 70 U, while men were treated with 60, 70, or 80 U. Using this variable dosing technique, 85% of BoNT-A-treated patients were rated as treatment responders by a blinded evaluator after 30 days compared with 3% of placebo-treated patients (p < 0.001). Compared with patients in other studies who received 50 U to the glabella (the approved, on label dose), variably dosed patients tended to have improved efficacy and quicker onset without increased adverse events.
BoNT-A products are not bioequivalent and cannot be used interchangeably because of differences in unit potency and fundamental differences in how these units are measured. As randomized, double-blind, placebo-controlled clinical trials comparing the aesthetic efficacy of these products have not been performed, numerous studies have attempted to use surrogate endpoints to establish relative potency rations for these products. To date, these attempts have not been successful.12–14,23–28 The reason for this is simple: the dose-response curves of onabotulinumtoxinA and abobotulinumtoxinA are not parallel and therefore a single dosing ratio between the two products cannot be established throughout the range of doses used.
Patient selection
Decision-making details of selecting a patient for BoNT-A
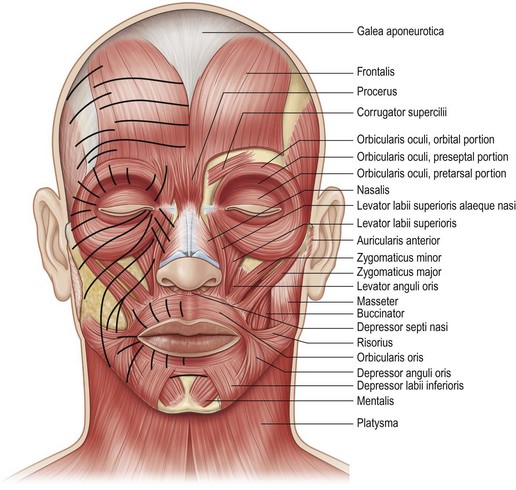
The absolute key to becoming a proficient BoNT-A injector instead of a mere technician is understanding the functional anatomy of the face. Anatomy textbooks show the location of different facial muscles and their origins and insertions. While these texts allow for small and expected anatomic variations, they cannot prepare the clinician for the overwhelming differences in functional anatomy between individuals, or even different sides of the same face. Even though all individuals have the same mimetic muscles, their smile patterns vary depending on which muscles dominate within the group.29 Even within a single muscle, different portions of that muscle can dominate and severely alter animation (Fig. 3.1).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree