Chapter 7
The Trunk and Urogenital System
- Open abdomen
- Perineal reconstruction
- Hypospadias
- Bladder exstrophy-epispadias complex
- Ambiguous genitalia
- Vaginal agenesis
- Penile reconstruction
- Further reading
Open abdomen
- An abdominal wall defect created by intentionally leaving an abdominal incision open at the completion of surgery, or by opening or re-opening the abdomen for abdominal compartment syndrome.
- Also known as a ‘laparostomy’.
- Abdominal wound dehiscence is a major post-operative complication with high mortality.
- Abdominal wall tissue loss following trauma or tumour also produces significant defects.
- Also known as a ‘laparostomy’.
Anatomy
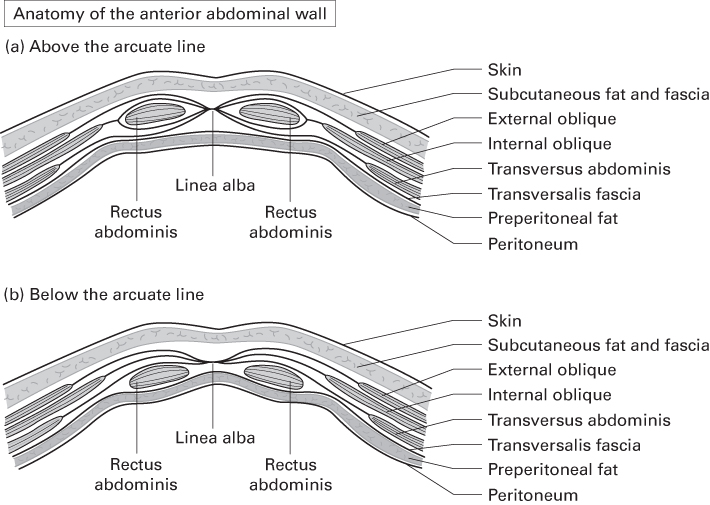
- In general terms, the anterior abdominal wall is composed of:
- Skin
- Subcutaneous fat within Camper’s fascia
- Scarpa’s fascia (below the level of the umbilicus only)
- Sub-Scarpa’s fat
- Anterior rectus sheath centrally, aponeuroses of the oblique muscles laterally
- Muscles
- Posterior rectus sheath (above the arcuate line only)
- Transversalis fascia
- Preperitoneal fat
- Peritoneum.
- Skin
Muscles
- Longitudinally orientated, centrally placed muscles:
- Rectus abdominis (RA)
- Pyramidalis.
- Flat, laterally placed muscles:
- External oblique (EO)
- Internal oblique (IO)
- Transversus abdominis (TA).
- Rectus abdominis (RA)
- Aponeuroses of EO, IO and TA fuse lateral to the lateral edge of RA to form the linea semilunaris.
- They divide into anterior and posterior laminae to enclose RA in a fascial sheath—the rectus sheath—before fusing again in the midline as the linea alba.
- The posterior lamina is deficient below the level of the ASIS; its free edge is the arcuate line.
- The arcuate line is approximately ⅓ the distance from umbilicus to pubic symphysis.
- Superior to the arcuate line, the anterior rectus sheath comprises the aponeurosis of EO and the anterior leaf of IO aponeurosis.
- The posterior rectus sheath comprises the posterior leaf of IO and TA aponeurosis.
- Inferior to the arcuate line, all three of these muscles’ aponeuroses pass anterior to RA.
- The posterior surface of RA therefore lies directly on transversalis fascia.
- Muscles have segmental nerve supply, usually T6/T7–T12.
- EO is also supplied by the iliohypogastric nerve (T12, L1).
- IO is also supplied by the iliohypogastric and ilioinguinal (L1) nerves.
- EO is also supplied by the iliohypogastric nerve (T12, L1).
- All nerves run in the neurovascular plane between IO and TA.
Arterial supply
- Segmental intercostal and lumbar vessels
- Superior epigastric arteries
- Superficial and deep inferior epigastric arteries
- Superficial and deep circumflex iliac arteries
- Superficial external pudendal arteries.
- Superior epigastric arteries
Pathogenesis
- The abdomen may be left open in these circumstances:
- Damage control surgery for trauma
- Intra-abdominal sepsis
- Excessive visceral oedema that precludes direct closure
- Abdominal wound dehiscence.
- Following decompression of abdominal compartment syndrome
- Intra-abdominal hypertension is defined as >12 mmHg.
- Abdominal compartment syndrome is defined as >20 mmHg with organ dysfunction.
- Intra-abdominal pressure is usually measured with an intravesical probe.
- Intra-abdominal hypertension is defined as >12 mmHg.
- Damage control surgery for trauma
Classification
- Björck et al. propose this classification of open abdomen:
- Grade 1: Open abdomen without adherence between bowel and abdominal wall or fixity of the abdominal wall (lateralisation).
- A: clean
- B: contaminated.
- A: clean
- Grade 2: Open abdomen with developing adherence/fixity.
- A: clean
- B: contaminated.
- A: clean
- Grade 3: Open abdomen complicated by fistula formation.
- Grade 4: Frozen open abdomen with adherent/fixed bowel that cannot be closed surgically, with or without fistula.
- Grade 1: Open abdomen without adherence between bowel and abdominal wall or fixity of the abdominal wall (lateralisation).
Management of the open abdomen
Three phases:
- Temporary abdominal closure
- Patient optimisation
- Definitive closure.
Temporary abdominal closure
- Purpose of temporary closure:
- Protect intestines
- Maintain a sterile (or at least clean) environment
- Avoid fluid and temperature loss.
- Protect intestines
- Methods of temporary abdominal closure:
- Skin-only closure using towel clips
- Silo technique, using non-adherent plastic sheets to wrap around intestines
- Temporary mesh
- Intraperitoneal packing
- Negative pressure wound therapy
- Various proprietary devices.
- Negative pressure wound therapy
- Skin-only closure using towel clips
Patient optimisation
- Patients are often critically ill.
- Optimisation maximises chances of successful reconstruction.
- Summarised by the ‘SNAP’ principle:
- Sepsis control—both intra-abdominal infection and systemic inflammatory response.
- Nutrition—usually requires supplemental enteral or parenteral feeding.
- Anatomy—defining the defect by pre-operative imaging.
- Planning—determining the type and extent of reconstruction required.
- Sepsis control—both intra-abdominal infection and systemic inflammatory response.
Definitive closure
- Small wounds may be amenable to delayed primary closure or healing by secondary intention.
- May be necessary to skin graft directly on to bowel or granulation tissue to minimise protein losses.
- Delayed reconstruction of the myofascial layer may be required months later.
- Patients unfit for reconstructive surgery are managed with an abdominal binder.
Reconstruction of the myofascial layer
- Prosthetic
- Bioprosthetic
- Autologous.
Prosthetic reconstruction
- Materials may be meshed or non-meshed; absorbable or non-absorbable.
- Meshed materials allow continued drainage of the abdominal cavity.
- Granulation can grow through the mesh to permit delayed skin grafting.
- Alternatively, mesh can be covered with omentum and skin grafted.
- Necessitates leaving a small abdominal defect for the omentum pedicle.
- Absorbable meshes are associated with higher rates of fistula and late hernia compared to polypropylene meshes, e.g. Marlex®.
Bioprosthetic reconstruction
- Acellular dermal matrix (ADM) is popular for abdominal hernia repair.
- Tends to form fewer adhesions with bowel compared to prosthetic materials.
- Certain ADMs incorporate by regeneration and are replaced by native tissue.
- They can therefore be used in infected wounds with relative impunity.
- ADMs generally lose integrity when used as ‘bridge grafts’ for ventral hernias, with high recurrence rates.
Autologous reconstruction
- Fascia lata, used as a graft or pedicled tensor fasciae latae flap.
- Can also be transferred as part of an anterolateral thigh (ALT) flap.
- Muscle flaps from local or distant sources:
- RA, either as a transposition or turnover flap.
- EO, for upper abdominal defects.
- IO, for lower abdominal defects.
- Latissimus dorsi, rectus femoris, vastus lateralis and gracilis may also be used, either pedicled or free.
- Components separation
- A technique described by Ramirez that allows greater advancement of myofascial layers towards the midline for direct closure.
- Releasing incisions are made at pre-determined points in the deep fasciae:
- EO aponeurosis is divided from the rectus sheath just lateral to the linea semilunaris.
- RA is separated from the posterior rectus sheath by incising the medial edge of the sheath and freeing the muscle.
- EO aponeurosis is divided from the rectus sheath just lateral to the linea semilunaris.
- Allows bilateral advancement of rectus muscles and anterior rectus sheath:
- 5 cm in the epigastrium
- 20 cm at the umbilicus
- 6 cm in the suprapubic region.
- 5 cm in the epigastrium
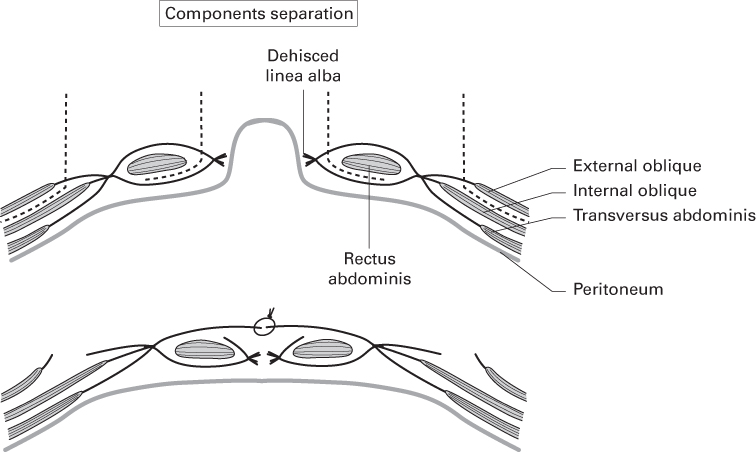
Source: Ramirez et al. (1990). Reproduced with permission of Elsevier.
- Tissue expansion
- Adjacent myofascial tissue can be expanded prior to advancement.
- Expander placement has been described between EO and IO and between IO and TA.
- Adjacent myofascial tissue can be expanded prior to advancement.
Post-operative complications
- Relatively common:
- Wound dehiscence
- Enterocutaneous fistula
- Hernia
- Infected prosthetic mesh—usually requires removal
- Seroma.
- Wound dehiscence
Perineal reconstruction
Anatomy
- The perineum is the region of the trunk inferior to the pelvic diaphragm.
The pelvic diaphragm
- Formed by the two levator ani muscles and two coccygeus muscles.
- Levator ani is composed of several parts:
- Puborectalis
- Pubococcygeus
- Iliococcygeus
- Levator prostatae or levator vaginae.
- Puborectalis
- These muscles form a hammock between pubis, coccyx and lateral pelvic walls.
- This keeps the pelvic contents within the pelvis.
- Puborectalis muscles unite posterior to the anorectal junction to form a muscular sling, creating the angle between rectum and anal canal.
Boundaries
- Perineum boundaries:
- Pubic symphysis and inferior pubic rami
- Ischial rami and ischial tuberosities
- Sacrotuberous ligaments and coccyx.
- Pubic symphysis and inferior pubic rami
- An imaginary transverse line joining the anterior ends of the ischial tuberosities divides the perineum into two areas:
- Urogenital triangle anteriorly, containing female external genitalia, or, in males, the root of the scrotum and penis.
- Anal triangle posteriorly, containing the anus.
- Urogenital triangle anteriorly, containing female external genitalia, or, in males, the root of the scrotum and penis.
The urogenital triangle
- The urogenital diaphragm is a muscular sheet attached to the sides of this triangle.
- Pierced by the urethra and, in the female, the vagina.
- The anterior and posterior parts of the diaphragm are formed by the deep transverse perineal muscles.
- The middle part is formed by the sphincter urethrae muscle.
- Tough fascia overlies (i.e. is superficial to) the urogenital diaphragm, termed the perineal membrane.
- Superficial to this membrane is the superficial perineal pouch, containing:
- Bulbospongiosus muscle
- Ischiocavernosus muscle
- Superficial transverse perineal muscle.
- In the female:
- These muscles are less well developed
- The bulbospongiosus is pierced by the vagina.
- The middle part is formed by the sphincter urethrae muscle.
The perineal body
- Lies at the midpoint of the line separating urogenital from anal triangles.
- It is the tendinous centre of the perineum where several muscles attach, including levator ani and anal sphincters.
The anal triangle
- Comprises the anus, external anal sphincter, levator ani and ischioanal fossae.
- The ischioanal fossae contain:
- Internal pudendal vessels.
- Pudendal nerve, supplying most perineal sensation.
- This neurovascular bundle runs in Alcock’s canal—a fascial tunnel overlying obturator internus in the lateral ischioanal fossa.
- Internal pudendal vessels.
Regional arterial anatomy
- Rich blood supply from two main sources:
- Femoral artery, giving superficial and deep external pudendal arteries.
- Internal iliac artery, giving internal pudendal and inferior gluteal arteries.
- Femoral artery, giving superficial and deep external pudendal arteries.
- Branches anastomose freely, allowing design of multiple flaps with robust blood supply.
Perineal skin
- Perineum contains both specialised and non-specialised skin.
- The skin is mobile to accommodate the full range of hip joint movement.
- Also accommodates defaecation, sexual intercourse and childbirth.
Pathology
- Perineal defects most commonly result from:
- Resection of gynaecological, colorectal and urological malignancy.
- Debridement of sepsis, particularly Fournier’s gangrene.
- Inflammatory bowel disease, e.g. Crohn’s disease and ulcerative colitis.
- Trauma, e.g. open pelvic fractures.
- Resection of gynaecological, colorectal and urological malignancy.
VIN, VAIN, AIN and invasive malignancy
- VIN, VAIN and AIN are potentially premalignant lesions associated with human papilloma virus (HPV) 16 and 18, smoking and immunosuppression.
- VIN is vulval intraepithelial neoplasia.
- VAIN is vaginal intraepithelial neoplasia.
- AIN is anal intraepithelial neoplasia.
- Immunisation against HPV may help prevent most of these lesions.
- Diagnosis is made on biopsy of suspicious areas.
- Dysplastic changes are graded similarly to cervical intraepithelial neoplasia (CIN I, II, III).
- High-grade dysplasia is thought to progress to invasive squamous carcinoma in many patients.
- VIN is vulval intraepithelial neoplasia.
Treatment of intraepithelial neoplasia
- Gold standard treatment of VIN is local excision.
- 12–17% of VIN excision specimens contain invasive squamous cell carcinoma (SCC).
- 40–60% of VIN lesions progress to invasive SCC.
- 12–17% of VIN excision specimens contain invasive squamous cell carcinoma (SCC).
- Alternatives to surgery include topical imiquimod and laser.
- Response rates are lower than with surgery; requires close follow-up.
- Treatments for VAIN include:
- Observation (particularly for localised VAIN I).
- Ablation with CO2 laser or cautery.
- Surgical excision (particularly for multifocal disease or VAIN III).
- Radiotherapy, usually delivered as brachytherapy (particularly for recurrent or multifocal VAIN III).
- Observation (particularly for localised VAIN I).
- Treatments for AIN are still being evaluated, but include:
- Observation (particularly for AIN I)
- Topical imiquimod
- Laser or cautery
- Surgical excision (particularly for AIN III).
- Observation (particularly for AIN I)
Treatment of gynaecological invasive malignancy
- Management is planned by a multi-disciplinary team (MDT) in a gynaecological cancer centre.
- Most cases are SCCs; vulval melanomas and vaginal adenocarcinomas are rarer.
Vulva
- SCC usually treated by excision, unless the patient is unfit for surgery.
- Primary treatment with radiotherapy can be used in such patients.
- Tumours are excised with 1cm margins, including any areas of adjacent VIN.
- Larger resections may require a defunctioning colostomy.
- Elective inguinal lymph node dissection may be required.
Vagina
- SCC often treated primarily with chemoradiotherapy.
- High posterior tumours may require surgical treatment.
- Advanced stage and recurrent tumours also require surgery due to a high incidence of fistulation into bowel or bladder.
- Surgery often involves pelvic exenteration, leaving significant dead space.
Treatment of anorectal invasive malignancy
- Management is planned by a colorectal MDT.
- The 2011 Anal Cancer Position Statement by the Association of Coloproctology of Great Britain and Ireland states that plastic surgical input should form part of the anal cancer MDT when reconstruction is considered.
- The delayed healing rate of perineal wounds following direct closure is 40–70%.
- This decreases to 15–25% with flap reconstruction.
Anus
- Primary treatment of anal SCC is chemoradiotherapy.
- Surgery is done for salvage if the tumour is unresponsive or recurrent.
- Usually involves abdomino-perineal resection (APR) of distal rectum and anal skin.
Rectum
- Low rectal tumours (predominantly adenocarcinomas) usually require APR.
- Permanent end-colostomy is formed.
- Depending on disease stage, adnexal structures may also be excised:
- Posterior vaginal wall
- Bladder
- Prostate and seminal vesicles.
- Posterior vaginal wall
- Multivisceral involvement requires either posterior or total pelvic exenteration.
- Coccyx or sacrum may also be excised.
- Neo-adjuvant chemoradiotherapy is indicated for advanced tumours.
Defect assessment
Volume deficit
- Superficial lesions may be reconstructed with skin grafts or thin local flaps.
- Significant dead space may allow descent of intestines into the pelvis—so-called perineal hernia.
- A bulky flap is required in such circumstances.
Radiotherapy
- Irradiated fields cause these problems:
- Preclude the use of local tissues for reconstruction.
- Increase incidence of wound-healing problems.
- Increase risk of fistulation.
- Preclude the use of local tissues for reconstruction.
Diversion
- When faecal and urinary diversion is planned, stoma location on the abdominal wall should be discussed with the MDT if RA is to be used for reconstruction.
Function
- Consider whether sexual function, faecal and urinary continence can be restored.
Principles of reconstruction
- Perineal reconstruction is indicated in these circumstances:
- Extensive skin loss
- Vaginal resection
- Pelvic or perineal dead space
- Excision of the pelvic floor
- Excision after radiotherapy.
- Extensive skin loss
- Reconstruction requires importation of vascularised, non-irradiated tissue of the required type, with the aims of:
- Managing dead space.
- Providing a substitute for the pelvic floor.
- Reconstructing the vagina.
- Managing dead space.
Flap options
- Pedicled flap options are plentiful due to the rich blood supply.
- External pressure exerted during lying, sitting and walking makes free tissue transfer riskier than in other areas.
- Commonly used flaps:
- Fasciocutaneous
- Lotus petal
- Superior gluteal artery perforator (SGAP)
- Inferior gluteal artery perforator (IGAP)
- Posterior thigh
- ALT
- Pudendal-thigh (‘Singapore’ flap)
- Lotus petal
- Myocutaneous
- Vertical rectus abdominis myocutaneous (VRAM)
- Inferior gluteal artery myocutaneous (IGAM)
- Gracilis
- Vertical rectus abdominis myocutaneous (VRAM)
- Omentum
- Colon.
- Fasciocutaneous
Lotus petal
- So-named because the possible skin paddles fan out from the midline like a lotus flower.
- In practice, the skin paddle does not have to look like a lotus petal and is designed to fit the defect.
- The flap option that lies in the buttock crease is most commonly used.
- In practice, the skin paddle does not have to look like a lotus petal and is designed to fit the defect.
- Based on perforators near the midline of the perineum.
- Perforators usually come from the internal pudendal artery as it traverses the ischioanal fossa.
- The density of anastomoses in this region makes identification of a named vessel inconsequential.
- The flap can be transposed or advanced as a V-Y.
- The density of anastomoses in this region makes identification of a named vessel inconsequential.
SGAP
- The superior gluteal artery is a branch of the internal iliac artery.
- Emerges from the pelvis superior to piriformis, deep to gluteus maximus.
- Surface marking is ⅓ along a line drawn from posterior superior iliac spine (PSIS) to the apex of the greater trochanter.
- Perforator location in some individuals may make it difficult to reach all perineal defects.
IGAP
- The inferior gluteal artery (IGA) is a branch of the internal iliac artery.
- Emerges from the pelvis inferior to piriformis, deep to gluteus maximus.
- Surface marking is ½ along a line joining PSIS and ischial tuberosity.
- Travels inferolaterally between greater trochanter and ischial tuberosity.
- While deep to gluteus it gives many myocutaneous perforators to inferior buttock skin.
- Also gives a descending branch—the basis of the posterior thigh flap.
Posterior thigh
- Based on the descending branch of the IGA.
- Can be raised as a large transposition flap based on this.
- Alternatively, most of the posterior thigh can be elevated as a large V-Y advancement flap based on IGA and profunda femoris perforators.
- Profunda perforators are on a line joining ischial tuberosity to lateral femoral condyle.
- The flap is sensate—incorporates the posterior cutaneous nerve of the thigh.
ALT
- Based on the descending branch of the lateral circumflex femoral artery.
- Tunnelled medially, deep to sartorius and rectus femoris, to reach the perineum.
- Femoral nerve branches to rectus femoris should be preserved.
- Vastus lateralis can be included for additional bulk.
Pudendal-thigh (Singapore or Wee flap)
- Fasciocutaneous flap based on the posterior labial branch of internal pudendal artery.
- Sensory supply is from the posterior labial branch of the pudendal nerve.
- Designed on non-hair-bearing skin in the groin crease, lateral to labia majora.
- The flap’s base is posterior, at posterior fourchette level.
- For vaginal reconstruction, bilateral flaps are raised and tunnelled to the midline.
- Sutured together outside the body to form an ‘inside-out’ tube.
- Pushing the tube into the pelvis inverts it, so the skin lines the neo-vagina.
- Sutured together outside the body to form an ‘inside-out’ tube.
VRAM
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







