Chapter 15 The skin bank
![]() Access the complete reference list online at http://www.expertconsult.com
Access the complete reference list online at http://www.expertconsult.com
![]() IN THIS CHAPTER
IN THIS CHAPTER ![]() PowerPoint Presentation Online
PowerPoint Presentation Online
History
The first skin autograft was described by Reverdin1 in 1871 and the use of allograft skin as a clinical method for wound coverage soon followed.2 In 1874, Thiersch published a report about a small series of patients on whom he had used partial-thickness skin grafts.3 This led to extensive trials of harvesting extremely thin grafts, leaving some of the surface epithelium behind to aid in donor site healing. Results from the use of these small thin grafts, known as ‘Thiersch grafts,’ ‘pinch grafts,’ ‘epidermis grafts,’ or ‘razor grafts’ were generally so unsatisfactory for resurfacing large areas that they were typically limited to the treatment of small ulcerated wounds. The first successful use of allogeneic skin for burn wound coverage was reported by Girdner4 in 1881. Five years later, Thiersch described the histologic anatomy of skin engraftment which popularized the clinical use of split-thickness skin grafts.3
Storage of human skin did not begin until the early 1900s, when Wentscher5 reported his experience with the transplantation of human skin that had been refrigerated for 3–14 days; however, it was not until the 1930s that blood and tissue banking took their place in the clinical practice of medicine. The clinical utility of allograft skin in burn wound coverage was first described in 1938 when Bettman6 reported his success in the treatment of children with extensive full-thickness burn injuries. Webster7 and Matthews8 later described the successful healing of skin autografts stored for 3 weeks at 4–7°C; however, it was not until 1949, following the establishment of the United States Navy tissue bank, that modern day skin banking began.
The establishment of skin banking signaled the beginning of significant research related to the processing, preservation, and storage of human tissues. Baxter9 explored the histologic effects of freezing on human skin and discovered that the formation of ice crystals caused the destruction of skin architecture. This was followed in 1952 by the pioneering research of Billingham and Medawar10 who demonstrated that skin could be effectively cryopreserved using glycerol. Soon afterwards, Taylor11 was able to demonstrate that the addition of glycerol to storage solutions decreased ice crystal formation in frozen tissues. These advancements permitted Brown12 and Jackson13 to popularize the use of allogeneic human skin grafts as biologic dressings for extensive burns and denuded tissue. By 1966, Zaroff14 had reported the 10-year experience using allograft skin in the treatment of thermally injured patients at the Brooke Army Medical Center. In this report, he described the mechanical and physiologic advantages of allograft skin as a biologic dressing. In 1966, Cochrane15 reported the first successful use of frozen autologous skin grafts following controlled-rate freezing in 15% glycerol and rapid rewarming prior to implantation. This was followed by Morris’16 report demonstrating the beneficial effects of using allogeneic skin in the treatment of infected ulcers and other contaminated wounds and Shuck et al.’s17 report suggesting the potential use of allogeneic skin in the treatment of traumatic wounds based upon their Vietnam War experience. These increased uses of allograft skin led to further research into the beneficial effects of allograft skin on wound healing, including its association with a reduced incidence of bacterial infections18,19 and the stimulation of wound bed neovascularization.20
Bondoc and Burke21 are credited with the establishment of the first functional skin bank in 1971. Their experience with allograft skin led to a report of successful burn wound excision and allografting with temporary immunosuppression in children with extensive injuries.22 Today, allograft skin remains an ideal temporary cover for extensive or excised cutaneous or soft tissue wounds, particularly when sufficient autograft skin is not available or when temporary wound coverage is desired.
Clinical uses of allograft skin
Coverage of extensive full-thickness wounds
The increasing use of allograft skin in specialized burn care centers has been one of the driving forces behind the growth and development of skin banks in the United States. The general indications for its use in wound management are listed in Box 15.1. Allograft skin possesses many of the ideal properties of biologic dressings and plays a major role in the surgical management of extensive wounds when autologous tissue may not be immediately available (Box 15.2). It reduces evaporative water loss and the exudation of protein-rich fluids, prevents wound desiccation, and suppresses microbial proliferation. Wound pain is lessened and is associated with better patient compliance with occupational and physical therapy. By restoring the physiologic barrier at the wound surface, the allografts reduce heat loss through the wound and mitigate the hypermetabolic response to burn injury. The frequent and unpredictable demand for allograft skin in specialized burn care centers has prompted the growth and development of local and regional skin banks throughout the world.
Box 15.1 Indications for allograft skin use in wound management
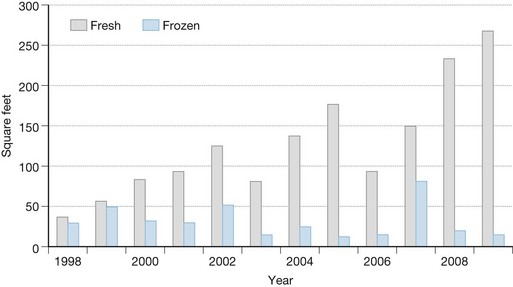
Fresh allograft skin represents the Gold Standard for all biologic dressings employed for temporary wound closure based upon a number of its distinctive properties compared to cryopreserved skin (Box 15.3). Its availability is critically important for the surgeon faced with the need to provide immediate coverage of large excised burn wounds. Allogeneic grafts are best applied unmeshed (or minimally expanded) in order to maximize their ability to temporarily close the wound. Fresh allografts become well vascularized, stimulate neovascularization in the underlying wound bed, and prepare the recipient sites for permanent coverage with autologous skin. In addition, fresh allografts tolerate modest wound contamination and adhere better to the freshly excised subcutaneous fat than do cryopreserved grafts. The allogeneic skin is usually removed once the patient’s donor sites have healed sufficiently for reharvesting or once autologous cultured skin is available for permanent wound closure. Figure 15.1 depicts the quantitative use of allograft skin and, in particular, the increased use of fresh refrigerated allograft skin, in thermally-injured children treated at the Shriners Hospital for Children in Cincinnati, Ohio since 1998.
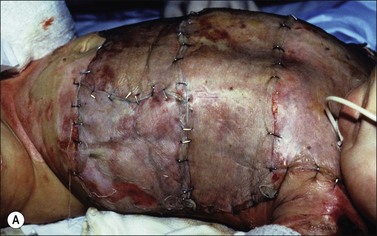
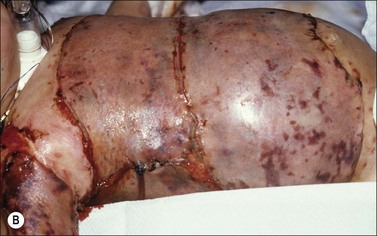
When fresh allograft is not available, cryopreserved skin is an excellent alternative for temporary wound coverage. Although frozen cryopreserved skin generally has less measurable viability than fresh skin, it is currently difficult to maintain continuous and ample stores of fresh skin beyond 14 days. It has therefore been standard skin banking practice to cryopreserve allograft skin within 7–10 days of recovery if it is not going to be utilized within the time period that viability can be maintained. Regardless of whether the allografts are fresh or cryopreserved, wide (mesh) expansion of allograft skin is rarely performed because reepithelialization of the interstices by allogeneic epidermis is uncommon. Figure 15.2 demonstrates the difference in appearance and vascularization of cryopreserved and fresh, refrigerated skin.
The emergence of Integra® dermal regeneration template for the treatment of excised burn wounds has been significant and in some burn centers has replaced or decreased the use of allograft skin in patients with extensive full-thickness burn injuries. Heimbach et al. reported the results of a randomized multicenter clinical trial comparing Integra® and other grafting materials.23 When the artificial dermis was compared to allograft, there was no difference in the ‘take’ rates; however, ‘take’ was not defined in the manuscript and it is unclear whether there were equivalent rates of adherence and/or vascularization. Furthermore, it is unclear if the allografts applied were fresh or cryopreserved, although it is most likely that the allografts were frozen as the refrigeration of allograft skin was not common skin banking practice at the time of the study. A subsequent retrospective study performed at our burn center and skin bank 10 years later indicated that fresh, refrigerated allograft had a better rate of engraftment than the dermal regeneration template.24
Coverage of widely meshed skin autografts
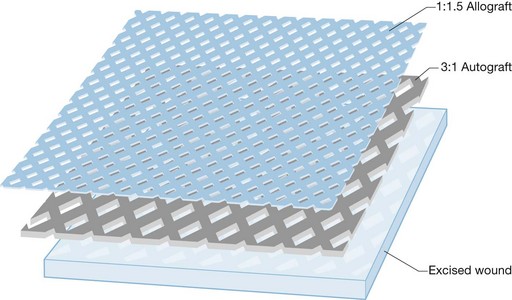
Another use of allograft skin has been its application as an overlay on top of widely expanded, meshed autologous skin grafts (Fig. 15.3). This technique was originally described utilizing meshed allograft25 and provides immediate, as well as both temporary and permanent wound closure. For this reason, most burn surgeons who currently perform overlay allografting utilize non-meshed or non-expanded mesh allografts. This affords better protection of the underlying wound bed from desiccation and microbial contamination as this tissue is potentially exposed by the interstices of the widely meshed autograft until autologous epithelialization is complete. While this technique may play a role in the management of massive excised full-thickness injuries, it should be used with discretion since many surgeons have expressed concern that the overlying allograft may induce an inflammatory rejection response that can retard the rate of reepithelialization of the underlying autografts. Some have therefore advocated the use of lyophilized allograft for this purpose as it is less viable and less antigenic.26
Template for delayed application of keratinocytes
The clinical use of cultured epidermal autografts (CEA) in the care of burn patients was first described by O’Connor et al.27 in 1981. Since that time, there have been numerous reports supporting its use as a permanent skin replacement for patients with extensive full-thickness burn injuries. This methodology has not been without problems, however, with many authors describing variable take rates and instability of the grafts. Cuono first advocated the use of allogeneic skin with CEA, allowing the allograft skin to vascularize before removing the antigenic epidermal layer by dermabrasion.28 Hickerson et al. reported results on five burn patients demonstrating over 90% CEA take on the allogeneic dermis and supple, durable grafts up to 4 years postoperatively.29
The past decade or so has also witnessed the development of an acellular allogeneic dermal matrix (AlloDerm®) as a template for the simultaneous application of thin split-thickness autografts.30 The potential advantage of the dermal template is reasoned to be the use of a thinner autologous skin graft resulting in more rapid donor site healing and reduced morbidity. A recently completed multicenter clinical trial demonstrated equivalence of this technique with a standard split-thickness meshed autograft; however, autograft take rates were somewhat lower than that for controls and varied from center to center.31 In addition, the allogeneic dermal grafts measured only 36–116 cm2 and were only evaluated up to 180 days post-grafting. AlloDerm® has also been used as a template for CEA; however, there have been only a few anecdotal reports related to this potential application.
Micrografting techniques
Chinese surgeons have proposed the use of micrografts using both autologous and allogeneic skin.32 This technique involves the mincing of autologous skin into pieces less than 1 mm in diameter. These micrografts are then used to seed the dermal surface of large sheets of allogeneic skin prior to transplantation onto the excised burn wound. As the autologous epidermal cells propagate on the wound surface, the allograft skin gradually separates in a manner similar to that observed with the overlay technique. This method, while resulting in an effective skin expansion ratio approaching 1 : 18, has been shown to be associated with severe wound contraction that is often worse than that noted following the application of meshed skin autografts.
Potential disadvantages of allograft use
Infection
Allograft skin has been reported to cause bacterial infection.33 It is therefore imperative that skin banks perform microbial cultures prior to releasing the tissue for transplantation. In fact, the American Association of Tissue Banks (AATB) Standards34 require that skin be discarded if pathogenic bacteria or fungi are present. This is particularly important given the immunocompromised status of the potential recipient and the potential for developing wound sepsis following such contamination.
There have also been reports of viral disease transmission by skin allografts. In 1987, Clarke reported what was thought to be the transmission of HIV-1 to a burn patient from an HIV-positive donor;35 however, the results of donor testing were not known prior to skin use. Moreover, the recipient, who had a number of risk factors for HIV, had not been tested prior to receiving the allograft. To date, there have been no other reported cases of HIV or hepatitis transmission from skin allografts.
Kealey et al. have reported the transmission of cytomegalovirus (CMV) from cadaver skin allografts.36 Because nearly 23% of the CMV-negative patients seroconverted, they recommended the use of CMV-negative allograft skin for seronegative burn patients. Plessinger et al. reviewed 479 consecutive skin donors and found 63% of this predominantly adult donor pool to be CMV-positive.37 They reasoned that while tissue from seronegative donors would be ideal for use in seronegative patients, such a practice would significantly limit the availability of fresh allograft skin for most thermally-injured patients. In addition, while there is good evidence to support the transmission of CMV by allograft in burn patients, there is little evidence that CMV seroconversion is clinically significant or affects outcome in thermally-injured patients.38,39 Furthermore, Herndon and Rose38 reiterated that the benefits of using cadaver allograft skin for the treatment of burn patients clearly outweigh the small risks associated with CMV seroconversion. At present, most burn surgeons and skin banks recommend that the decision regarding the use of allograft skin from CMV-positive donors should be made by the burn/transplanting surgeon.
Rejection
While demonstrating many characteristics of an ideal wound covering, allograft skin contains Langerhans cells which express class II antigens on their surface. These cells reside in the epidermis of the skin and are ultimately rejected as the result of an immunologic rejection response. This typically results in an acute inflammatory reaction and can lead to wound infection. Vascularized allogeneic skin grafts typically remain intact on the wound of a burn patient for 2–3 weeks although there have been reports of allograft skin survival for up to 67 days due to the inherent immunosuppression of extensive burn injury.40
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree