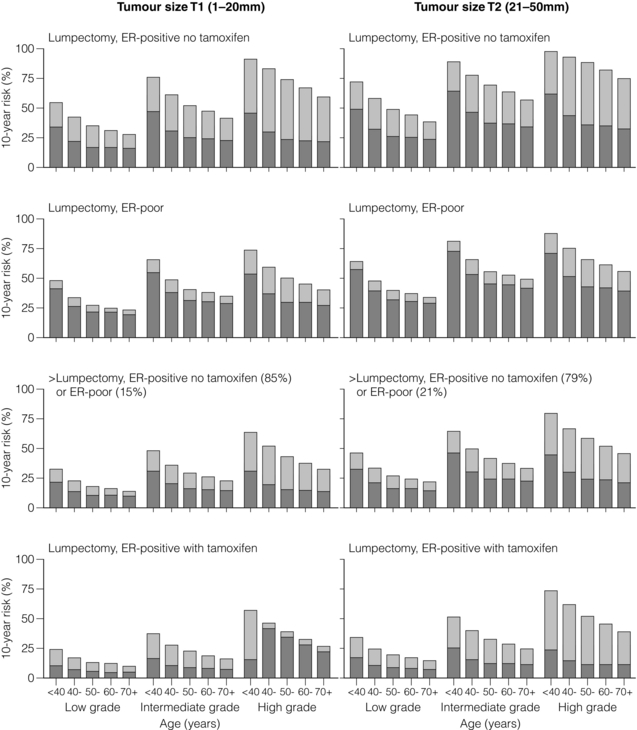
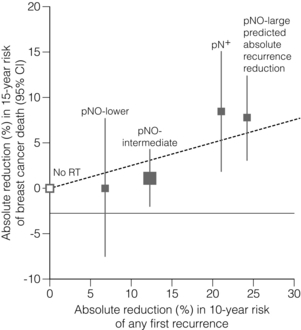
15 Three-dimensional CT planning has improved dose homogeneity both within the breast and/or to regional nodal areas while reducing dosage to critical normal tissues, particularly the lungs, heart and brachial plexus. In addition, breathing-adapted gating techniques have reduced cardiac irradiation.1 Achieving a homogeneous dose distribution is challenging in the breast because its contour changes in both cranio-caudal and sagittal planes. Homogeneity is poorer in the upper and lower regions of the breast, resulting in ‘hot spots’ in areas of the breast distant from the central axis.2 Tangential beam plans can be optimised to ensure that the volumes of the breast exceeding 105% of the prescribed dose are minimised. With IMRT the X-ray beam is dynamically collimated to modify its fluence, allowing a therapeutic dose to be ‘painted’ to the breast/chest wall and peripheral lymphatics while minimising dosage to critical adjacent structures. The ‘field-in-field’ technique (the addition of supplementary photon fields) reduces ‘hot spots’ such as the inframammary fold and the thin breast tissue close to the nipple–areola complex.2,3 In principle, DCIS should be as sensitive as invasive breast cancer (IBC) to irradiation. However, its role in the conservative management of DCIS is less firmly established than for IBC and use of adjuvant RT varies across the UK with no clear standard of care.5 All these trials used a dose fractionation schedule of 50 Gy in 25 fractions over 5 weeks. The EORTC trial6 randomised 1010 women with clinically or mammographically detected DCIS ≤ 5 cm in size to wide local excision alone or wide local excision plus whole breast irradiation (50 Gy in 25 fractions over 5 weeks). The 10-year local relapse-free rate was 85% with RT compared to 74% with surgery alone (hazard ratio (HR) 0.53, P < 0.001). In situ local recurrence rates were 7% and 13% respectively and invasive rates were also 7% and 13%. In the NSABP B-17 trial7 818 patients were randomised to +/− whole-breast radiotherapy (WBRT) after lumpectomy. RT reduced the local relapse rate from 16.8% to 7.7% (relative risk (RR) 0.38, 95% confidence interval (CI) 0.25–0.59; P < 0.00001). In a recent update of the trial with 10-year follow-up, invasive recurrence within the ipsilateral breast was associated with a slightly higher risk of death. In contrast, recurrence of DCIS was not.8 The UK/ANZ trial9 recruited 1701 patients treated by BCS. Patients were randomised into four treatment groups (BCS alone, BCS + RT, BCS + tamoxifen and BCS + RT + tamoxifen). Approximately 90% of patients were ≥ 50 years and screen detected. Median follow-up was 53 months. Local recurrence rates were 22%, 8%, 18% and 6% respectively. Adjuvant RT was associated with a significant reduction for both DCIS and IBC ipsilateral recurrence (HR 0.38, P < 0.0001). Radiotherapy reduced the risk of recurrence by 64% for DCIS (P = 0.0004) and by 55% for invasive year cancer (P = 0.01). The Swedish DCIS trial (SweDCIS)10 randomised 1067 patients after wide excision for DCIS to WBRT or no WBRT. There was a risk reduction of 16% in ipsilateral events (in situ or invasive carcinoma) at 10 years from RT (95% CI 10.3–21.6%) and a relative risk of 0.40 (95% CI, 0.30–0.54%). The effect of RT in women was lower in women younger than 50, but there was a substantial benefit in women > 60 years. Results of the four trials were pooled in a Cochrane review11 and showed that the risk of ipsilateral invasive recurrence was halved by radiotherapy at 10 years (HR 0.50, 95% CI 0.32–0.76) with about 50% of the ipsilateral breast tumour recurrence (IBTR) being invasive and 50% DCIS. In a subgroup analysis according to age (</> 50 years, presence or absence of comedo necrosis and tumour size >/< 10 mm) all subgroups derived benefit from RT. Women > 50 years appeared to experience a greater reduction in recurrence than compared to younger women (HR 0.35 (> 50) vs. 0.67 (< 50)). What limits interpretation, as others have pointed out,5 is that none of the trials were designed prospectively for subgroup analyses. In addition, there have been criticisms of the quality of the DCIS trials, summarised in a review,13 and problems with these trials include deficiencies in radiological–pathological correlation, measurement of tumour size,14 routine imaging of pathology specimens, postoperative imaging, definition and classification of lesions,7 definition of tumour-free margins,15 consistency in inclusion/exclusion criteria, randomisation procedures and insufficient statistical power to detect small differences in survival.12 Attempts have been made to define a subset of patients from whom postoperative radiotherapy might be omitted. In the E5194 non-randomised study of wide local excision alone in 711 patients with low – or intermediate-grade DCIS ≤ 2.5 cm or high-grade ≤ 1 cm with margins ≥ 3 mm with median follow-up of 6.7 years, the 5-year ipsilateral recurrence rate was 6.1%.16 A continued increase in the rate of IBTR beyond 5 years is of concern and long-term follow-up will be needed to determine if omission of radiotherapy is safe. The absolute benefits of radiotherapy on local control are greater in high-grade compared to low-grade DCIS. The thresholds for recommending radiotherapy vary widely internationally. In some centres radiotherapy is confined to patients with high-grade DCIS, whereas other women with all grades of DCIS are treated following BCS on the basis17 that there is no group that does not benefit. One of the arguments for a more conservative approach to selection is that these patients are subject to the same risks of radiation-induced morbidity (including cardiac damage, rib fractures and pneumonitis) as patients with invasive breast cancer. In some groups of women with DCIS mortality rates are < 1% and so this encourages a more cautious approach in recommending adjuvant breast irradiation. That said, the risks, for example, of cardiopulmonary morbidity have been substantially reduced by the use of three-dimensional CT planning. A multi-institution retrospective study comparing wide local excision alone, wide local excision and whole-breast radiotherapy among 373 patients ≤ 45 years showed a reduced risk of relapse at 10 years in patients treated with a boost dose (86%) compared to no boost (72%).18 However, this study was potentially subject to selection bias, with the possibility that higher risk patients were more likely to have received a boost.19 The role of the boost and of hypofractionation is currently under investigation in the international BIG 3-07 trial. Patients with non-low-risk DCIS are randomised after whole-breast irradiation to a boost of 16 Gy in eight fractions or no boost. An external beam dose fractionation regimen (the standard of 50 Gy in 25 fractions over 5 weeks) or a hypofractionated regimen of 42.5 Gy in 16 daily fractions are options that are included in the randomisation or can be selected in the trial. The primary end-point is time to local recurrence. The overall impact of radiotherapy on local recurrence and survival is best appreciated from the 5-yearly updates of the Oxford overview of randomised trials of radiation after both breast-conserving surgery and mastectomy. The conclusions have changed dramatically since the first overview in 1978, which showed an adverse effect of RT on survival after mastectomy,21 largely due to the adverse effects of excessive cardiac irradiation from now obsolete orthovoltage techniques. However, in 2005, the first evidence emerged of the beneficial effect of a reduction in local recurrence from RT on the overall survival23 of 42 000 women in 78 randomised trials. This ratio was confirmed in a more recent meta-analysis confined to over 10 000 women of trials of breast-conserving surgery with or without adjuvant whole breast irradiation.24 It should be noted that the primary end-point in the latter overview has been changed from previous radiotherapy overviews to include any first recurrence, whether this is locoregional or metastatic. The overall impact of adjuvant radiotherapy is an impressive 50% reduction in the risk of any first recurrence. However, the absolute benefits of radiotherapy are less in older lower-risk patients (Fig. 15.1). The 10-year risk of any recurrence was reduced by radiotherapy by 15.7% (35% to 19.3%; 95% CI 13.7–17.7, 2P < 0.00001) and the 15-year mortality fell from 25.2% to 21.4% (an absolute reduction of 3.8%; 95% CI 1.6–6.0, 2P = 0.00005). In 7287 pN0 patients, the equivalent risk was reduced from 31% to 15.6%, which is a 15.4% absolute reduction (95% CI 13.2–17.6, 2P < 0.00001) with a 3.3% absolute reduction in mortality from 20.5% to 17.2%. In pN1 (1050 patients) the 10-year reduction in risk of recurrence was greater (21.2%; 95% CI 14.5–27.9, 2P < 0.00001) from 63.7% to 42.5% with an 8.5% reduction in 15-year breast cancer mortality (95% CI 1.8–15.2, 2P = 0.01) from 51.3% death to 42.8%. The risk of any first recurrence at 10 years was influenced by tumour size, age, grade, use of tamoxifen, oestrogen receptor (ER) status and extent of local breast surgery (Fig. 15.2). The proportional reduction in first recurrence is similar irrespective of age. However, the absolute reduction in risk is much less for women > 70 years. Most recurrences (75%) were locoregional and were higher in the no RT groups (25%) compared to 8% in the RT arms. The dominant effect on locoregional recurrence from RT was in the first year but there was a lesser but still substantial impact up to 9 years. Figure 15.1 Absolute 10-year risks (%) of any (locoregional or distant) first recurrence with and without radiotherapy (RT) following breast-conserving surgery (BCS) in pathologically node-negative women by patient and trial characteristics, as estimated by regression modelling of data for 7287 women. Bars show 10-year risks in women allocated to BCS only. Dark sections show 10-year risks in women allocated to BCS plus RT, light sections show absolute reduction with RT. ER, oestrogen receptor. Reprinted from The Lancet. Early Breast Cancer Trialists’ Collaborative (EBCTCG). Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: meta-analysis of individual patient data for 10 801 women in 17 randomised trials. Lancet 2011; 378 (9804):1707–16. With permission from Elsevier. Figure 15.2 Absolute reduction in 15-year risk of breast cancer death with radiotherapy (RT) after breast-conserving surgery versus absolute reduction in 10-year risk of any (locoregional or distant) recurrence. Women with pNO disease are subdivided by the predicted absolute reduction in 10-year risk of any recurrence suggested by regression modelling (pNO large ≥ 20%, pNO intermediate 10–19%, pNO lower<10%). Vertical lines are 95% CIs. Sizes of dark boxes are proportional to amount of information. Dashed line: one death from breast cancer avoided for every four recurrences avoided. pNO, pathologically node negative; pN+, pathologically node positive. Reprinted from The Lancet. Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: meta-analysis of individual patient data for 10 801 women in 17 randomised trials. Lancet 2011; 378(9804):1707–16. With permission from Elsevier. Six hundred and thirty-six women, 70 years or older with T1, N0, M0 breast tumours, were randomised after breast-conserving surgery and tamoxifen to whole-breast irradiation or no further treatment. The difference in local recurrence was 3% at 5 years (4% vs. 1%) in favour of adjuvant irradiation.25 Breast oedema, skin fibrosis and pain were all more frequent in the irradiated group. In an accompanying editorial27 the value of radiotherapy was questioned given the small difference in local recurrence. However, no axillary surgical staging procedure was included in this study so it is possible that the some higher risk, node-positive patients were enrolled. In addition, as the authors acknowledge, the trial was underpowered. It is well recognised that there is a persistent pattern of local recurrence of up to 1% per year at least up to 10 years.28 This concern is validated by the update of the CALGB trial showing that at a median follow-up of 10.5 years, the difference in local recurrence has increased to 7% (9% vs. 2%).26 Of the 43% of patients who had died only 7% of deaths were due to breast cancer, reflecting the competing risks of death from non-breast cancer causes, predominantly vascular, in older patients with breast cancer. Two additional trials shed light on the possibility of the omission of RT in some patients. The Italian 55-75 trial29 randomised 749 women with T (< 2.5 cm) N0/1, M0 breast cancer to whole-breast irradiation (50 Gy in 2-Gy fractions) after quadrantectomy and systemic therapy. For N0 patients, sentinel lymph node biopsy (SLNB) was undertaken. SLNB-positive patients were additionally treated by axillary clearance. This trial is not directly comparable to the CALGB trial since it was not exclusive to older patients and higher risk patients with one to three involved nodes were included as well as hormone receptor-positive and -negative tumours. At a median follow-up of 53 months, the cumulative incidence of IBTR was 2.5% in the surgery-alone arm and 0.7% in the surgery plus radiotherapy arm. The smaller difference in IBTR (1.8%) in the Italian 55-75 trial compared to the CALGB trial largely reflects the greater volume of breast tissue resected by quadrantectomy compared to lumpectomy. The PRIME II trial,30 currently in the follow-up phase, included over 1300 patients ≥ 65 years with T < 3 cm pathologically axillary node-negative breast cancer after breast-conserving surgery (minimum 1 mm clear margin) and adjuvant endocrine therapy who were randomised to whole-breast irradiation (40–50 Gy) or no whole-breast radiotherapy. Other randomised trials of breast-conserving surgery with or without postoperative radiotherapy that have included but were not limited to older patients do not provide an answer to the question of the omission of postoperative radiotherapy after breast-conserving surgery. In a Canadian trial,31 age was an independent risk factor with a higher locoregional recurrence rate (LRR) in women over the age of 50. However, a low-risk group with an LRR < 10% could not be identified. In the Milan III trial, women over the age of 55 years had a lower risk of recurrence (3.8% vs. 8.8% for the whole population).32 In the Scottish conservation trial,33 there was a trend to a lower recurrence rate with age, particularly between 60 and 70 years. No difference in local recurrence was observed in the NSABP B-06 trial for women older or younger than 50 years.34 The upper age of the trial, however, is not stated. It should be noted, however, that 5-year local recurrence rates in more recently published studies of breast conservation are falling to around 3%.36 In part this is due to better imaging, screening, surgery and systemic therapy, particularly the introduction of aromatase inhibitors. So the absolute benefits in local control from whole-breast irradiation for older patients are likely to diminish. There is a need to identify better markers of women who are genuinely at low risk of local recurrence to better define clinical ‘low-risk’ categories and then provide level I evidence to underpin this approach. There is level I evidence that a boost of irradiation to the site of excision after breast-conserving surgery improves local control in older as well as young patients. The EORTC boost trial randomised over 5000 T1/2, N0, NIM0 patients after breast-conserving surgery with clear margins and whole-breast irradiation (50 Gy) to a boost of 16 Gy in eight fractions or no boost. The original analysis37 at 5 years of follow-up showed that the benefit in local control was only statistically significant in women under the age of 50. However, at 10 years of follow-up,38 all age groups were shown to benefit, although the reduction in local recurrence (7.3% vs. 3.8%) was only 3.5% in the over 60 years of age group. It might be assumed that the quality of life of older patients treated with postoperative radiotherapy would be worse than for patients treated by breast-conserving surgery and adjuvant endocrine therapy alone due to the burden of attending for several weeks of radiotherapy. However, there is good evidence that adjuvant radiotherapy is well tolerated by the majority of older patients.39,40 The only trial to address this issue is the PRIME I trial. It randomised 255 T1/2, N0, M0 patients treated by breast-conserving surgery and endocrine therapy to whole-breast radiotherapy (40–50 Gy) or no further therapy. There was no overall difference in global quality of life measured by the EORTC breast QoL measures41 at 5 years.
The role of adjuvant radiotherapy in the management of breast cancer
Advances in radiotherapy planning and delivery
The role of adjuvant radiotherapy in ductal carcinoma in situ (DCIS)
Is there a role for a boost dose after whole-breast irradiation for DCIS?
Role of adjuvant radiotherapy in invasive breast cancer
Is there a subgroup of patients from whom postoperative radiotherapy can be omitted?
Breast boost after breast-conserving surgery for invasive breast cancer
Impact of adjuvant whole-breast radiotherapy on quality of life
Plastic Surgery Key
Fastest Plastic Surgery & Dermatology Insight Engine






