The Rickettsioses, Ehrlichioses, and Anaplasmoses: Introduction
|
Rickettsial diseases are curable infections that, if unrecognized, can be readily lethal. Early nonspecific symptoms can mimick benign viral illnesses and should be considered in any patient who presents with constitutional symptoms, fever, headache, and a characteristic petechial rash. The advent of modern molecular technologies, including genetic analysis, has allowed for significant taxonomic reclassification of the rickettsiae including moving the family Bartonella (see Chapter 192) and the genus Coxiella out of the order Rickettsiales to the orders Rhizobiales and Legionellales, respectively. “Rickettsiae” now includes a polyphyletic group of microorganisms in the class Proteobacteria, comprising species belonging to the genera Rickettsia, Orientia, Ehrlichia, Anaplasma, and Neorickettsia. Several non-rickettsial agents that were historically included in the group of infections loosely termed the rickettsioses remain incorporated in the discussion of rickettsial disease herein to reflect that precedent.1
Historically rickettsiae and Rickettsia-like organisms (e.g., Coxiella burnetii) were endemic pathogens, however the rise of international travel has allowed for the spread of rickettsial infections to nonendemic areas. Rickettsia prowazekii and C. burnetii, agents of epidemic typhus and Q fever, respectively have emerged as potential agents of bioterrorism.2
Historical Aspects
![]() The rickettsioses have had profound effects on civilization, particularly during times of war or famine. The first contemporary account of rickettsial disease appeared during the civil wars of Granada in 1489–1490, where a typhus-like disease killed 17,000 Spanish soldiers. The eighteenth century physician James Lind astutely recognized that the vector of epidemic typhus was carried on the bodies of men and clothes. In 1909, Charles Nicolle identified the body louse as the vector for epidemic typhus. During World War I and the Russian Revolution, epidemic typhus caused 3 million deaths. Howard Tyler Ricketts, for whom the genera was named, is credited with first demonstrating the organism (now known to be R. prowazekii) in typhus patients and infected lice. Ironically, Ricketts ultimately died of typhus in Mexico in 1910. Before his death, however, Ricketts also confirmed that the wood tick was a vector of Rocky Mountain spotted fever (RMSF). S. Burt Wolback at Harvard University Medical School is credited with identifying the organism that causes RMSF within the endothelial cells of vasculitic lesions.
The rickettsioses have had profound effects on civilization, particularly during times of war or famine. The first contemporary account of rickettsial disease appeared during the civil wars of Granada in 1489–1490, where a typhus-like disease killed 17,000 Spanish soldiers. The eighteenth century physician James Lind astutely recognized that the vector of epidemic typhus was carried on the bodies of men and clothes. In 1909, Charles Nicolle identified the body louse as the vector for epidemic typhus. During World War I and the Russian Revolution, epidemic typhus caused 3 million deaths. Howard Tyler Ricketts, for whom the genera was named, is credited with first demonstrating the organism (now known to be R. prowazekii) in typhus patients and infected lice. Ironically, Ricketts ultimately died of typhus in Mexico in 1910. Before his death, however, Ricketts also confirmed that the wood tick was a vector of Rocky Mountain spotted fever (RMSF). S. Burt Wolback at Harvard University Medical School is credited with identifying the organism that causes RMSF within the endothelial cells of vasculitic lesions.
![]() The earliest serologic test for rickettsiae was based upon the work of Weil and Felix in 1915, who demonstrated that the serum of patients with typhus agglutinated certain strains of Proteus vulgaris. Hans Zinsser, who recognized Brill’s disease (later called Brill–Zinsser disease) as a recrudescent form of epidemic typhus, wrote:
The earliest serologic test for rickettsiae was based upon the work of Weil and Felix in 1915, who demonstrated that the serum of patients with typhus agglutinated certain strains of Proteus vulgaris. Hans Zinsser, who recognized Brill’s disease (later called Brill–Zinsser disease) as a recrudescent form of epidemic typhus, wrote:
![]() “Typhus is not dead. It will live on for centuries, and it will continue to break into the open whenever human stupidity and brutality give it a chance, as most likely they occasionally will.”4
“Typhus is not dead. It will live on for centuries, and it will continue to break into the open whenever human stupidity and brutality give it a chance, as most likely they occasionally will.”4
Rickettsiae
Rickettsiae are obligate, intracellular, Gram-negative bacteria. They are pleomorphic 0.3–1 μm coccobacilli composed of DNA and RNA and reproduce through binary fission. Rickettsiae are propagated by arthropod vectors that use mammals (and sometimes the arthropods themselves) as reservoirs of infection. Rickettsiae are separated into the spotted fever group and the typhus group on the basis of common genetics, immunologic patterns, and intracellular growth characteristics. The typhus group lives entirely within the cell cytoplasm, whereas the spotted fever group can reside within the cytoplasm or nucleus. Rickettsiae are differentiated by unique antigenic structures on cell surface proteins. Rickettsia rickettsii has two major surface proteins, outer membrane protein A (OmpA) and B (OmpB), which are the main targets for serological testing.
Infection occurs through arthropod-induced breaks in the skin allowing access of the pathogen to the blood and lymph. Spotted fever rickettsiae are injected into the host through the saliva of the feeding tick, whereas typhus group rickettsiae enter through the feces of infected human body lice or fleas. Manipulation of the bite site, a long attachment of the arthropod, and exposure to arthropod hemolymph during tick removal aid in the transmission of pathogenic organisms. The rickettsial organisms then spread via the hematogenous and lymphatic systems, attach to endothelial cell membranes, and are phagocytosed. Spotted fever group rickettsiae stimulate host cells to produce reactive oxygen species and cause actin polymerization that aid in bacterial extrusion. In contrast, typhus group rickettsiae replicate intracellularly until the host cell bursts.3 The severe clinical manifestations of rickettsial infection (e.g., hypovolemia, purpura, pulmonary and cerebral edema) are caused by proliferation of bacteria within the vascular endothelium resulting in a multifocal, systemic vasculitis and microvascular leakage.4
Rocky Mountain Spotted Fever (RMSF) was first recognized in 1896 in the Snake River Valley of Idaho and was originally called black measles because of the characteristic appearance of the rash. Caused by the tick-borne R. rickettsii, it is the most frequently reported rickettsial infection in the United States. Fatal outcomes have been reported in 5% of treated cases, and as high as 20% of untreated cases.5
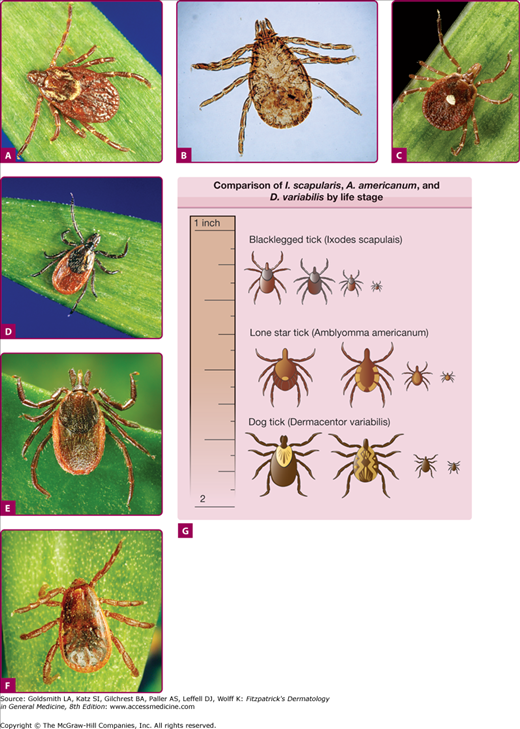
The vector largely responsible for RMSF in the eastern two-thirds of the United States is the American dog tick, Dermacentor variabilis, whereas the Rocky Mountain wood tick, Dermacentor andersoni, is prevalent in the Western United States (Fig. 199-1). RMSF is most prevalent in the Southeastern and South Central states, during spring and early summer. Historically, children younger than 10 years of age had the highest incidence of RMSF; however, surveillance data in the United States during 2003 demonstrate a higher age-specific incidence in persons aged 40–64 years.6 The principal epidemiologic characteristics of RMSF are outlined in Table 199-1.
Figure 199-1
Tick species responsible for transmission of Rickettsia, Ehrlichia, and Anaplasma. A. Dermacentor variabilis. B. Dermacentor andersoni. C. Amblyomma americanum. D. Ixodes scapularis. E. I. pacificus. F. Rhipicephalus sanguineus. G. Comparison of I. scapularis, A. americanum, and D. variabilis by life stage. (A US dime is shown to approximate relative size.) (From the Centers for Disease Control and Prevention (http://www.cdc.gov).)
Disease (Agent) | Primary Vector(s) | Epidemiology | Incubation Period | Risk Factorsa | Prognosis | First Line Treatment | Second Line Treatments |
|---|---|---|---|---|---|---|---|
Rocky Mountain spotted fever (Rickettsia rickettsii) | Dermacentor variabilis (American dog tick), Dermacentoandersoni (Rocky Mountain wood tick), Amblyomma americanum (lone star tick), Rhipicephalus sanguineus (brown dog tick), Amblyomma cajennense (Central and South America), Haemaphysalis leporispalustris ticks | Widespread in the United States, concentration in South Atlantic and South-Central states; Argentina, Brazil, Columbia, Costa Rica, Mexico, Panama, Canada Spring–Summer | >2–14 days | Males; adults 40–64 years, children <10 years; rural dwelling | Severe disease: G6PD deficiency (rapid decompensation, fulminant disease with higher incidence of necrosis), males, elderly, neurologic disease, hepatitis, jaundice, thrombocytopenia, acute renal failure, sulfa medications, delayed diagnosis and treatment. | Adultsb: Doxycycline 100 mg po q12h × 5–10 days Childrenc: Doxycyline 2.2 mg/kg po q12h × 5–10 days Pregnancyc: Doxycycline 100 mg po q12h for 5–10 days | Adults: Chloramphenicol, 50–75 mg/kg po q6h × 5–10 days or Tetracycline 500 mg q6h po × 5–10 days Children: Chloramphenicol, 12.5–25 mg/kg po q6h × 5–10 days Pregnancyd: Chloramphenicol |
Mediterranean spotted fever (Rickettsia conorii) | R. sanguineus (brown dog tick) | Endemic to Southern Europe, Middle East, Southwest Asia, India, and Africa Summer | 5–7 days | Exposure to dogs | Mild disease (mainly in children) has good prognosis. Severe disease “malignant Mediterranean spotted fever”: Alcoholics, elderly, G6PD deficiency, diabetes mellitus, heart disease, delayed diagnosis and treatment. | Adults: Doxycycline 200 mg po q12h × 1 day or 100 mg po q12h for 2–5 days Childrenc: Doxycyline 2.2 mg/kg po q12h × 5–10 days Pregnancy: Azithromycin 500 mg po daily × 3 days | Adults: Tetracycline 500 mg po q6h × 10 days or Ciprofloxacin 750 mg q12h for 8 days Children: Clarithromycin, 15 mg/kg/day divided q12h for 7 days or Azithromycin, 10 mg/kg/day daily × 3 days Pregnancyd: Chloramphenicol |
Rickettsialpox (Rickettsia akari) | Liponyssoides sanguineus (Allodermanyssus sanguineus) (house mouse, Mus musculus, mite) | United States (mainly eastern seaboard), South Africa, Korea, Ukraine, Croatia Sporadic | ∼ 7 days (eschar), ∼ 7–24 days (systemic symptoms and rash) | Males; urban dwellers (likely from mice exposure); intravenous drug users | Excellent. | Self-limiting | Doxycycline can be used for severe cases |
Endemic typhus (Rickettsia typhi; Rickettsia felis) | Xenopsylla cheopis (rat flea), Ctenocephalides felis (cat flea) | Southeast United States, Gulf region, Southern California; worldwide most cases in Africa (reported on all continents except Antarctica) Summer | 1–2 weeks (∼12 days) | Exposure to fleas, poverty, poor hygiene | Excellent prognosis with treatment. Complicated disease occurs with alcoholism, elderly, hematologic disorders, and exposure to sulfa-containing drugs. | Adults: Doxycycline 100 mg po q12h for 7–14 days Childrenc: Doxycyline 2.2 mg/kg po q12h × 5–10 days Pregnancyc,d: Chloramphenicol or Doxycycline | Adults: Chloramphenicol, 80–100 mg/kg/day po divided q6h Children: Chloramphenicol, 12.5–25 mg/kg po q6h for 5–10 days Pregnancy: Azithromycin |
Epidemic typhus (Rickettsia prowazekii) | Pediculus humanus var. corporis (human body louse), Neohaematopinus sciuropteri lice, Orchopeas howardii fleas | United States, Eastern Europe, Africa, Central and South America, China, Himalayan region Sporadic (increased in Winter) | ∼1–2 weeks (8 days, average) | Wartime, poor hygiene, natural disasters, cold weather | Good prognosis with treatment. Fever abates in 2 weeks (without treatment) and 48 hours (with treatment). Recovery of strength in 2–3 months | Adultse: Doxycycline 200 mg single dose (or until afebrile for 24 h) Childrenc: Doxycyline 2.2 mg/kg po q12h × 5–10 days Pregnancyc,d: Chloramphenicol or Doxycycline | Adults: Chloramphenicol, 80–100 mg/kg/day po divided q6h Children: Chloramphenicol, 12.5–25 mg/kg po q6h for 5–10 days Pregnancy: Azithromycin |
Human monocytic ehrlichiosis (Ehriichia chaffeensis) | A. americanum (lone star tick), D. variabilis (American dog tick), Ixodes pacificus ticks | South and Mid-Atlantic, North/South-Central United States, isolated areas of New Englandf Spring and summer (peak in May–July | 5–14 days | Males; adults >70 years | Poor prognosis: Immunosuppression (HIV, transplantation, glucocorticoids); cough, lymphadenopathy, diarrhea; use of trimethoprim-sulfamethoxazole. | Adults: Doxycycline 100 mg po q12h for 5–14 days Childrenc: Doxycyline 2.2 mg/kg po q12h × 5–14 days Pregnancyc: Doxycycline 100 mg po q12h for 5–14 days | Adults: Tetracycline 500 mg po q6h × 5–14 days or Rifampin 300 mg po q12h x7–10 days Children: Tetracycline 25–50 mg/kg/day po divided q6h × 5–14 days or Rifampin 10 mg/kg po q12h × 7–10 days Pregnancy: Rifampin 300 mg po q12h x7–10 days |
Human granulocytic anaplasmosis (Anaplasma phagocytophilum) | Ixodes scapularis (Eastern United States) and I Ixodes pacificus (Western United States), Ixodes ricinus (Europe) (black-legged ticks) | New England, upper Midwest United States, Mid-Atlantic, Northern California and Europeg Spring and summer (peaks in July and November | 5–21 days | Males; adults 60–69 years | Poor prognosis: Elderly, marked lymphopenia, anemia, immunosuppression (HIV, transplantation, glucocorticoids); development of opportunistic infections (e.g., Aspergillosis, disseminated candidiasis, etc.). | Adultsh: Doxycycline 100 mg po q12h for 5–14 days Childrenc,h: Doxycyline 2.2 mg/kg po q12h × 5–14 days Pregnancyc,h: Doxycycline 100 mg po q12h for 5–14 days | Adults: Tetracycline 500 mg po q6h × 5–14 days or Rifampin 300 mg po q12h x7–10 days Children: Tetracycline 25—50 mg/kg/day po divided q6h × 5–14 days or Rifampin 10 mg/kg po q12h × 7–10 days Pregnancy: Rifampin 300 mg po q12h x7–10 days |
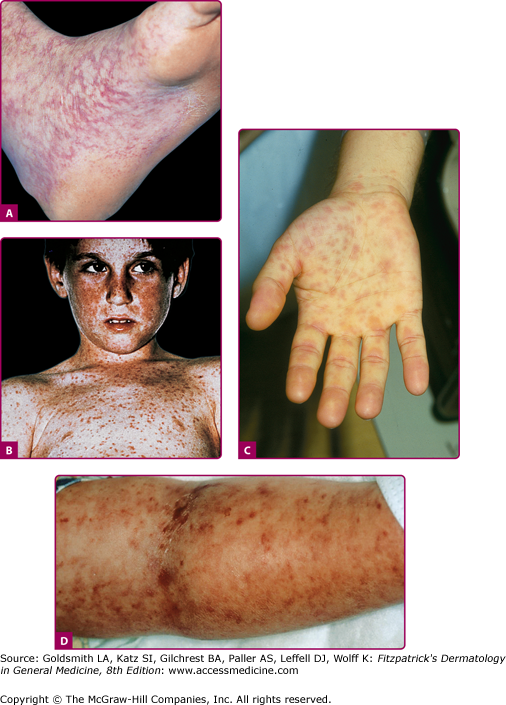
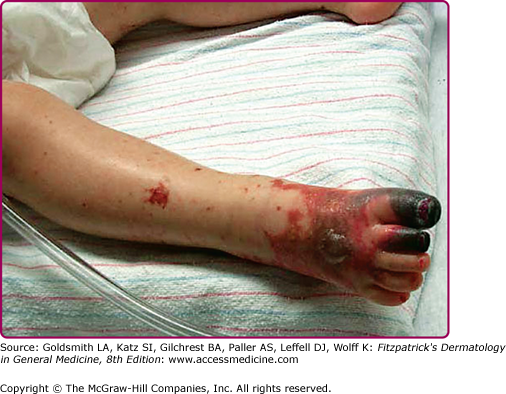
A high index of suspicion is important to the diagnosis of RMSF. One or two classic symptoms, outlined in Table 199-2, may be seen at presentation, but only about 60% of patients will have the complete clinical triad of fever [>39.5°C (102°F)], headache, and rash. Fever usually presents within the first 3 days of the illness, followed by a characteristic rash 2–4 days after the onset of fever. The rash usually starts on the wrists and ankles, spreading centripetally over the next 6–18 hours. Palms and soles are typically involved with relative sparing of the face.6 Cutaneous lesions are initially blanchable red macules that become papular and display evidence of petechiae or purpura (Fig. 199-2). Atypical “spotless” fever, seen in approximately 20% of cases does not imply milder disease, and is more common in the elderly and darker-skinned individuals.4 Periorbital edema, confusion, abdominal pain mimicking an acute abdomen, conjunctival injection, palatal petechiae, edema of dorsal hands, and calf pain are sometimes appreciated. Necrosis from overwhelming vasculitis is rare and preferentially occurs in peripheral locations such as the digits, penis, and scrotum (Fig. 199-3).7
RMSF | HME | HGA | |
|---|---|---|---|
Tropism |
|
|
|
Common initial signs and symptoms |
|
|
|
Rash |
|
|
|
Common laboratory abnormalities |
|
|
|
Systemic sequelae |
|
|
|
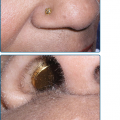
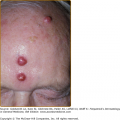
Figure 199-2
A. Early macular blanchable macules and papules on the ankle and sole. No truncal lesions were present at this time. B. Truncal and facial hemorrhagic macules and papules 7 days after the onset of the rash. C. Erythematous macular lesions on the palm may develop into a petechial rash that spreads centrally. (Reprinted from Collins SP: Cutaneous conditions. In: Atlas of Emergency Medicine, 2nd edition, edited by KJ Knoop, LB Stack, AB Storrow. New York, NY, McGraw-Hill, 2002, p. 382.) D. Petechial lesions on the arm of a child with fulminant Rocky Mountain spotted fever. (Used with permission from MMWR, Diagnosis and Management of Tick-borne Rickettsial Diseases: Rocky Mountain Spotted Fever, Ehrlichiosis, and Anaplasmosis—United States. March 13, 2006/55(RR04); 1-27, http://www.cdc.gov/mmwr.)
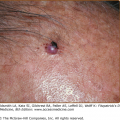
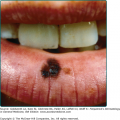
Figure 199-3
Gangrene of the toes in Rocky Mountain spotted fever. (Used with permission from Gary Marshall, MD and MMWR, Diagnosis and Management of Tick-borne Rickettsial Diseases: Rocky Mountain Spotted Fever, Ehrlichiosis, and Anaplasmosis—United States. March 13, 2006/55(RR04);1–27. http://www.cdc.gov/mmwr.)
Thrombocytopenia, anemia, mild hyponatremia, and mild transaminitis may be present. The white blood cell count is typically normal, however an increase in bands may be observed.6 Severe and life-threatening cardiac, gastrointestinal, hepatic, neurologic, ophthalmologic, renal, and pulmonary manifestations can occur with delayed or inadequate treatment. In patients with severe RMSF who survive the acute illness, long-term sequelae are usually the result of neurologic deficits or acral necrosis. In a case series by Buckingham et al, of 92 children diagnosed with RMSF, the median delay between seeking medical attention and antibiotic therapy was 6 days, with only 49% reporting a tick bite.8 Table 199-2 outlines the cutaneous and systemic manifestations of RMSF, as well as common laboratory abnormalities.
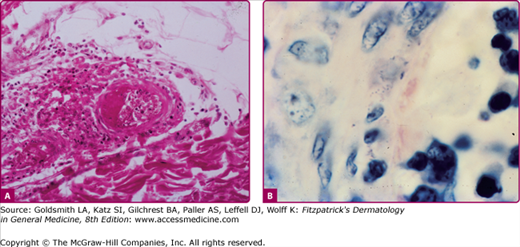
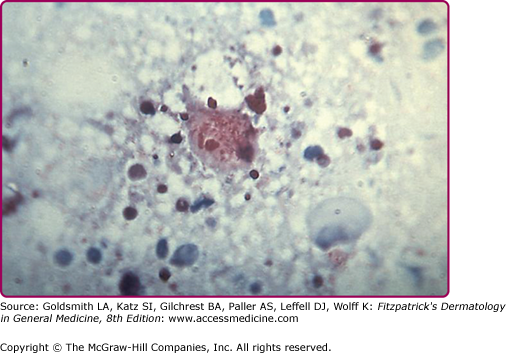
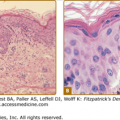
Histopathologic examination shows a septic vasculitis (Fig. 199-4). Early lesions demonstrate dermal edema with a predominantly perivascular lymphohistiocytic infiltrate and extravasated erythrocytes. Lymphohistiocytic vasculitis can progress to leukocytoclastic vasculitis. Basal cell vacuolization, lymphocytic exocytosis, fibrin thrombi, and capillary wall necrosis can also be appreciated. Immunohistology reveals positive staining for R. rickettsii in infected endothelial cells and tick hemolymph. (Fig. 199-5.) Most rickettsial diseases share a similar histology.
Serologic examination using the indirect immunofluorescence assay (IFA) is the gold standard for diagnosis of RMSF. It detects convalescent antibodies (at a diagnostic titer of ≥64 IgG and ≥32 IgM) but is seldom diagnostic before the seventh day of disease, and often not until far into the second week. An effective treatment for RMSF should begin by the fifth day of illness. It is vital to begin empiric therapy while awaiting serologies. Because of inferior sensitivity and specificity, the Weil–Felix test (agglutination of certain Proteus sp.) and complement fixation tests have been largely supplanted by newer diagnostic methods. Immunohistochemical staining of skin or organ tissue biopsy and polymerase chain reaction (PCR) may also confirm the diagnosis.9
When a patient presents without a rash, the differential diagnosis for RMSF is broad (Boxes 199-1 and 199-2). Macules and papules, petechiae, or purpuric lesions may sometimes develop in these diseases as well, further precluding the ability to distinguish them from RMSF on clinical grounds alone.
Most Likely Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|