The Pathogenesis of Keloids
Heather Woolery Lloyd
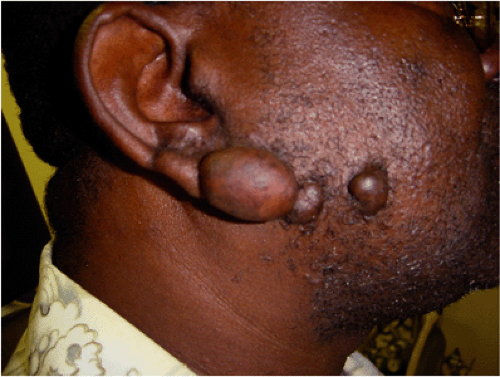
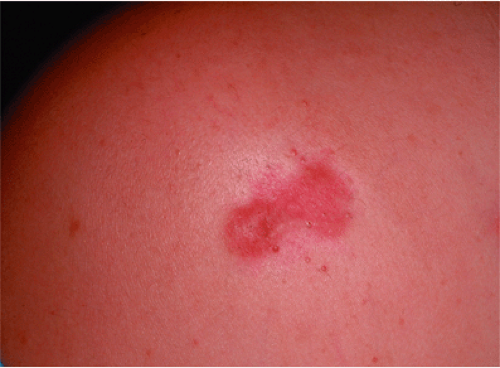
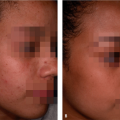
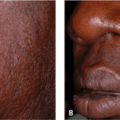
Darker racial ethnic groups have unique wound-healing characteristics to be considered when performing cosmetic procedures. The main concern with surgical procedures in patients with skin of color is the occurrence of keloids and hypertrophic scars. Keloids and hypertrophic scars are most common in darker racial ethnic groups and are most prevalent in African American, Hispanic, and Asian populations (Fig. 33-1 and Fig. 33-2). Statistics on the incidence of keloids in specific populations vary greatly; however, an incidence of 4.5% to 16% has been reported in black and Hispanic populations. Keloids are least common in Caucasians and albinos.1
Patients with keloids usually present in the second or third decade. There is an equal risk between women and men. Although most keloids occur sporadically, familial cases have been described. These cases appear to have an autosomal dominant pattern with incomplete penetrance and variable expression.2
Commonly affected sites for keloids include earlobes, shoulders, upper back, and chest. Postsurgically, they appear to be most common in areas of highest tension. Unlike hypertrophic scars, keloids rarely regress spontaneously. Once excised, they tend to recur. Common symptoms include pruritus and tenderness at the site of the keloid. These symptoms are most severe in new scars and tend to diminish with time.
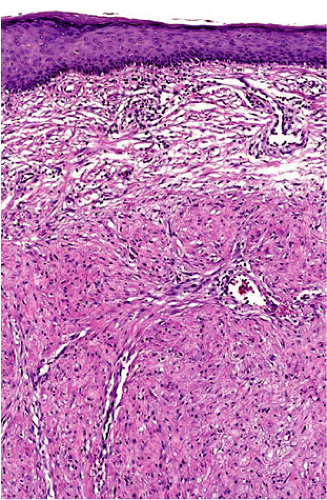
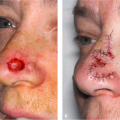
Histopathology of keloidal tissue reveals a thickened dermis consisting of hypereosinophilic, hyalinized collagen (Fig. 33-3). Few adnexal structures or elastic fibers are observed. Hypertrophic scars are more cellular than keloids, and hyalinized collagen is less prominent (Fig. 33-4).3
The mechanism behind keloid formation has not yet been elucidated; however, abnormal fibroblast metabolism and enhanced response to growth factors have been suggested. Other theories include aberrant apoptosis and abnormal epidermal-dermal interaction during wound healing. In this chapter, the many proposed mechanisms behind keloid formation will be explored. (Table 33-1).
There has been much discussion on the relationship between keloids and hypertrophic scars. Older studies often grouped these two entities and studied them together. More recently, an effort has been made to study these scars separately as two distinct disease entities. Keloid pathogenesis will be the main focus of this chapter to maximize clarity of the discussion.
Altered Growth Factors/Cytokines
Transforming growth factor beta
Transforming growth factor beta (TGF-beta) has been closely implicated in scar formation. Its role in keloid biology has been extensively studied, and it has been found to be profibrotic in wound healing. There are three isoforms of TGF-beta: TGF-beta1, TGF-beta2, and TGF-beta3. Of the three isoforms, TGF-beta1 is most abundant in all tissues and in wound fluid. In vitro studies demonstrate that TGF-beta1 and TGF-beta2 are profibrotic isoforms and promote scar formation.4 Interestingly, some studies suggest TGF-beta3 may have the opposite effect and may, in fact, reduce scar formation.5 Other data suggests that the ratio between TGF-beta3 and TGF-beta1 most affects scar formation.6
During wound healing, TGF-beta is first released by degranulating platelets. Other major cells involved in
wound healing that secrete TGF-beta include lymphocytes, macrophages, endothelial cells, epithelial cells, and fibroblasts. Once released, TGF-beta is involved in chemotaxis, angiogenesis, and extracellular matrix formation. It also stimulates dermal fibroblast proliferation and migration.4
wound healing that secrete TGF-beta include lymphocytes, macrophages, endothelial cells, epithelial cells, and fibroblasts. Once released, TGF-beta is involved in chemotaxis, angiogenesis, and extracellular matrix formation. It also stimulates dermal fibroblast proliferation and migration.4
Because of its clearly profibrotic role in wound healing, TGF-beta has been extensively studied in keloid formation. In vitro studies have shown that TGF-beta1 stimulates synthesis of keloid-derived fibroblasts more than it stimulates normal fibroblasts. TGF-beta also increases collagen synthesis in keloid fibroblasts more than in normal fibroblasts. These results suggest that keloid fibroblasts may have increased sensitivity to the effects of TGF-beta compared with normal fibroblasts.7
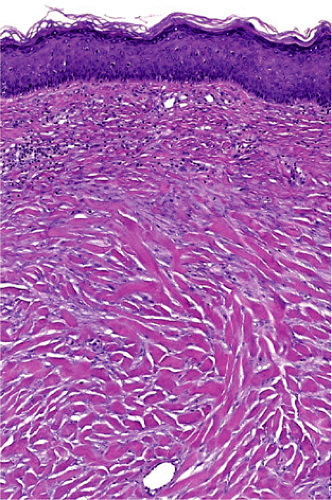 Figure 33-3 Hematoxylin, eosin stain of a keloid. Thickened dermis with hypereosinophilic and hyalinized collagen. (Courtesy of Pearl E. Grimes, MD and Jag Bhawan, MD) |
In addition to increased fibroblast and collagen synthesis, TGF-beta may also inhibit collagen degradation. Matrix metalloproteinases (MMPs) and plasminogen activator are the two main enzymes involved in breakdown of the extracellular matrix. Plasminogen activator is inhibited by plasminogen activator inhibitor 1 (PAI-1). MMP is inhibited by tissue inhibitor of metalloproteinase-1 (TIMP). TGF-beta up-regulates both PAI-1 and TIMP and, thus, may inhibit collagen degradation.8,9
Most studies clearly demonstrate the profibrotic role that TGF-beta plays in wound healing. Accordingly, multiple studies have examined the effect of anti-TGF antibodies on wound healing. Not unexpectedly, inhibition of TGF-beta with anti-TGF-beta antibodies in animal studies reduces scar formation.5,10
One study specifically examined the temporal effect of TGF-beta1, 2, and 3 on wound healing. In this study, anti-TGF-beta1, 2, and 3 were applied to rabbit ear wounds at different periods in wound healing. Anti-TGF-beta actually delayed wound healing when applied early to the wounds. Additionally, anti-TGF-beta applied early did not improve
the scar compared with untreated controls. This implies that TGF-beta is important and necessary for optimal wound healing in the first week after injury. However, anti-TGF-beta applied 1 week or more after injury resulted in reduced scar hypertrophy. Thus, early in wound healing, TGF-beta is necessary for optimal healing. However, after 1 week, the presence of TGF-beta may contribute to hypertrophic scar formation.11
the scar compared with untreated controls. This implies that TGF-beta is important and necessary for optimal wound healing in the first week after injury. However, anti-TGF-beta applied 1 week or more after injury resulted in reduced scar hypertrophy. Thus, early in wound healing, TGF-beta is necessary for optimal healing. However, after 1 week, the presence of TGF-beta may contribute to hypertrophic scar formation.11
TGF-beta clearly appears to be involved in abnormal scar formation; however, it does not appear to be the sole causative agent in keloids and hypertrophic scars. In fact, plasma levels of TGF-beta are no different in patients with keloids and controls. Additionally, common polymorphisms of the TGF-beta 1 gene are not associated with a risk of keloid disease.12 Although it appears that TGF-beta is important in understanding keloid biology, it does not appear to be the sole factor in keloid pathogenesis.
Platelet-derived growth factor
Platelet-derived growth factor (PDGF) is another profibrotic growth factor that has been implicated in keloid pathogenesis. In wound healing, PDGF acts both as a chemoattractant and as a mitogen for fibroblasts. Interestingly, in fetal (nonscarring) wounds, this growth factor disappears quickly. In scarring wounds there is prolonged expression of PDGF.13 Keloid fibroblasts also appear to be more responsive to PDGF’s profibrotic effects than normal fibroblasts. The enhanced PDGF response of keloid fibroblasts may be influenced by increased levels of PDGF alpha receptors on keloid fibroblasts.14
Table 33-1 Research and theories in keloid biology | |||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||||||
Connective tissue–derived growth factor
Connective tissue–derived growth factor (CTGF) is another tissue growth factor that has been implicated in
fibroproliferative disorders.15 CTGF is important for skeletal development, angiogenesis, cell adhesion, and cell migration.16 CTGF is also a downstream mediator of TGF-beta activity and is secreted by fibroblasts after activation by TGF-beta.17 In one study, CTGF expression was up-regulated in hypertrophic scar fibroblasts at baseline and after TGF-beta stimulation. In this study, a trend toward increased CTGF expression was also seen in keloid fibroblasts; however, this increased expression did not reach statistical significance.18



fibroproliferative disorders.15 CTGF is important for skeletal development, angiogenesis, cell adhesion, and cell migration.16 CTGF is also a downstream mediator of TGF-beta activity and is secreted by fibroblasts after activation by TGF-beta.17 In one study, CTGF expression was up-regulated in hypertrophic scar fibroblasts at baseline and after TGF-beta stimulation. In this study, a trend toward increased CTGF expression was also seen in keloid fibroblasts; however, this increased expression did not reach statistical significance.18
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree