Fig. 6.1
Illustration of design of the groin flap. Midline structures, the xiphoid and pubis, the inguinal ligament inferiorly and costal arch superiorly, and axillary line laterally are marked with a surgical marker. A flap designed within these boundaries will incorporate the superficial epigastric vessels in a reliable manner with a consistent zone of perfusion. It would be helpful to extend the inferior lateral portion of the flap slightly over the anterolateral thigh for ensuring inclusion of the vascular pedicle without jeopardizing it during inferior dissection (1 the femoral vessels, 2 the superficial epigastric vessels, 3 medial branch of the superficial epigastric artery, 4 lateral branch of the superficial epigastric artery, 5 the popliteal vessels, 6 saphenous vessels, 7 inguinal ligament, P pubis, X xiphoid, G Groin flap)
The surgical site should be sterilized using alcohol or betadine. It would be safer to perform the medial border incision first. From this incision the skin is raised and dissected laterally. This allows one to directly visualize the axial vessels from underneath. Medially and inferiorly dissection extends to include the groin fat pad. The incisions are then made along the remaining superior and lateral edges of the designed flap. The superior epigastric vessels usually need to be cauterized at the superior edge of the flap. Laterally, bleeding may occur from branches of the superficial epigastric vessels, which is easily controlled by a cautery. Inferiorly a plane should be created between the groin fat pad and overlying skin to protect the epigastric vessels diving into the fat pad. This is simply performed by careful dissection using a scissors. Then the inferior border of the flap is easily incised. The entire flap can be easily raised from cranial to caudal direction secondary to loose skin structure of the rat at a plane above the abdominal wall musculature. The femoral vessels should be identified to trace the superficial epigastric vessels. The entire flap is then freed from the wound bed and pedicled solely on the superficial epigastric vessels to create the axial pattern island groin flap model (Fig. 6.2). The flap can also be utilized as a free flap model revascularizing at the epigastric vessel level, which is technically challenging. For this purpose, in the groin region femoral vessels or in the neck the carotid artery and superficial jugular vein can be used for an end-to-side anastomosis. On the other hand, one may also utilize the entire groin flap using the larger caliber femoral vessels as the pedicle. In this way, not only one may increase the vessel size (femoral artery: 1.0 mm, femoral vein: 1.2–1.5 mm), but also a pedicle length of 2 cm or more can be obtained. This is easily achieved by meticulous dissection, isolation, and ligation or cauterization of various branches emerging with the femoral vessels both proximal and distal to the origin of the superficial epigastric vessels (Fig. 6.3).
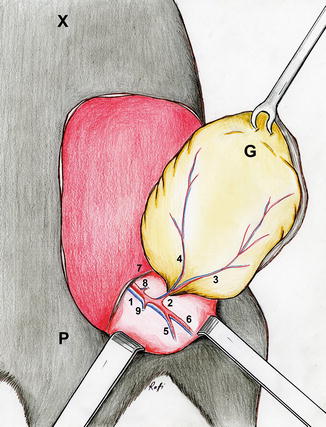
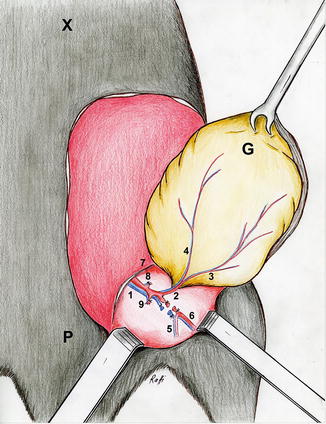

Fig. 6.2
Illustration: Island groin flap based on the superficial epigastric vessels (1 the femoral vessels, 2 the superficial epigastric vessels, 3 medial branch of the superficial epigastric artery, 4 lateral branch of the superficial epigastric artery, 5 the popliteal vessels, 6 saphenous vessels, 7 inguinal ligament, 8 the superficial circumflex iliac vessels, 9 the vascular branches to the gracilis muscle, P pubis, X xiphoid, G Groin flap)

Fig. 6.3
Illustration: The groin flap pedicled on the femoral vessels (1 the femoral vessels, 2 the superficial epigastric vessels, 3 medial branch of the superficial epigastric artery, 4 lateral branch of the superficial epigastric artery, 5 the popliteal vessels, 6 saphenous vessels, 7 inguinal ligament, 8 the superficial circumflex iliac vessels, 9 the vascular branches to the gracilis muscle, P pubis, X xiphoid, G Groin flap)
First, the femoral vessels are ligated and divided distally. Dissection is then continued proximally. The vascular branches to the gracilis muscle and the superficial circumflex iliac vessels are next to ligate and divide. Maximum attention should be paid not to damage pudic epigastric trunk and or external pudental vein right at the inguinal ligament since bleeding from these veins may be quite difficult to control. These large venous branches do not need to be dissected. The entire flap now pedicled on the femoral vessels can be harvested for replantation or used as a transplantation model (Fig. 6.4). The femoral vessels can be used as recipient vessels for revascularization [7] (Figs. 6.5, 6.6, and 6.7). Six to eight stitches using 10–0 or 11–0 microsuture are sufficient for arterial and venous anastomoses. The carotid artery and superficial jugular vein can be used as recipient vessels if the flap is transferred to the neck. The size of the femoral vessels and these recipient vessels permits end-to-end anastomosis.
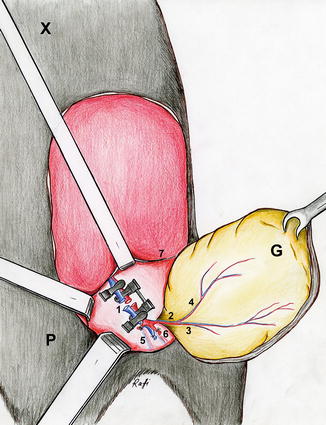
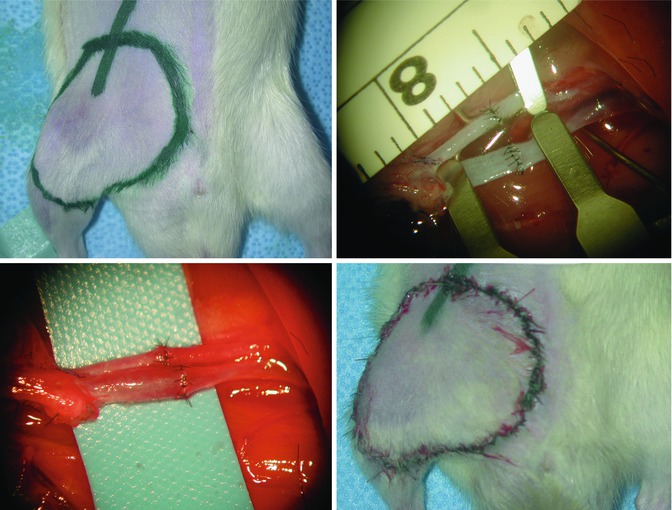
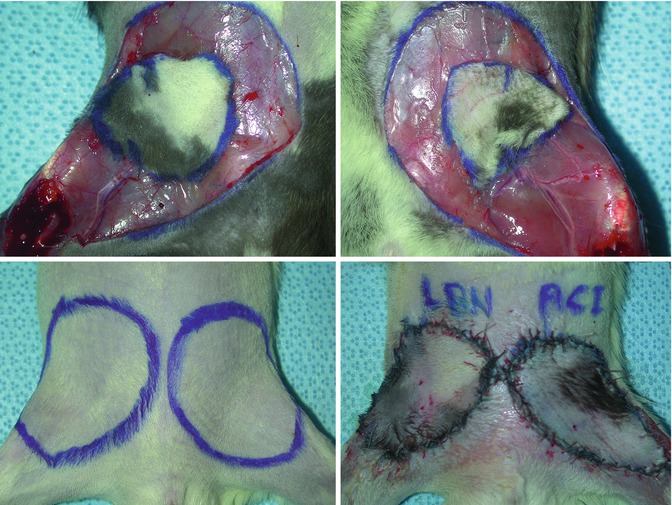
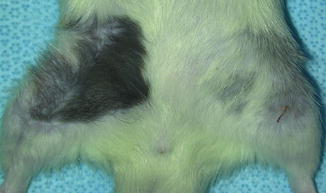

Fig. 6.4
Illustration: The groin flap as replantation model. Revascularization using the femoral vessels (1 the femoral vessels, 2 the superficial epigastric vessels, 3 medial branch of the superficial epigastric artery, 4 lateral branch of the superficial epigastric artery, 5 the popliteal vessels, 6 saphenous vessels, 7 inguinal ligament, P pubis, X xiphoid, G Groin flap)

Fig. 6.5
(left upper): Design of the groin flap on the right groin, (right upper): end-to-end arterial (above) and venous (below) anastomoses between femoral vessels for revascularization of the groin flap, (left lower): patent arterial (above) and venous (below) vascular anastomoses after microvascular clamps are removed (right lower): the groin flap is stitched into its place taking into account preoperative marking for right orientation

Fig. 6.6
(left upper): Groin flap prepared in a Lewis Brown Norway rat strain for transplantation, (right upper): groin flap prepared in an ACI rat strain for transplantation, (left lower): marking of groin skin bilaterally in a Lewis rat strain to be replaced by two groin transplants, (right lower): groin flap transplants in place after revascularization using femoral vessels in the recipient and donor animals

Fig. 6.7
Viable transplants at 50 days after transplantation under immunosuppression
Torsion and kinking of the pedicle and undue tension on the anastomotic site should be avoided. After restoration of perfusion, the flap is sutured back to its place in case of orthotopic replantation and transplantation or at a heterotopic site. A few key stitches are placed in the right orientation using interrupted 5–0 monofilament suture. This is followed by a running stitch to finalize the skin closure. Placement of marks around the periphery of the flap prior to skin incisions allows proper positioning of the flap. Autocannibalization is very common among rats. Therefore, rats should be individually housed and protective rat vest should be employed postoperatively to avoid autocannibalization [8].
Assessment of Flap Viability
One would expect a complete survival in a standard groin flap as described here. Nevertheless, the flap viability can be evaluated by inspection at about 1 week after surgery. However, depending on the particular project, surviving flap areas and non-viable zones, if any, can be evaluated separately. A variety of methods can be utilized for this purpose. As an example, the necrotic and viable areas on the groin flaps may be separately marked on transparent nylon sheets. These drawings are then transferred to a computer by the use of a standard PC scanner. With the help of a software program, the respective areas for viable and non-viable skin flaps (if any) can be calculated in cm2. Flap survival is also presented with percentages.
Another way of assessing the flap is by means of microangiography. Groin flaps can be studied angiographically to visualize the vascularization. The entire groin flap is carefully elevated as to not to damage the pedicle of the flap. The abdominal aorta is entered with a 24F cannula. The cannula is then directed to the right or left iliac artery and securely fixated with microsutures. Animals are sacrificed humanely by a lethal dose of pentobarbital anesthesia (100 mg/ml) administered intraperitoneally. The vascular tree is initially washed out with 50 cc isotonic sodium chloride solution. Through the same catheter, a mixture of 40 % barium sulfate (40 cc), 10 % gelatine (10 cc) and 50 cc saline solution is injected using a constant pressure syringe. The flap is then harvested. Radiopaque markers can be placed on the borders of the flaps to assist with orientation in microangiography [9]. Microangiographic pictures are taken at a 1:1 reproduction ratio using an x-ray machine with quality resolution (DMR, Senographe, General Electric, kV:24, MAS:8). Depending on the particular project, histological sections are harvested with or without special staining to assess various structures of interest.
Modifications of the Groin Flap
The potential neovascularization from the bed may contribute to survival of rat groin flaps. This issue may pose a risk that can potentially jeopardize results of a particular project. Placement of polythene sheeting under the flap as a barrier may be helpful. But it may be associated with undesired wound complications. Nishikawa and his associates [10] described the development of a modified rat groin flap which utilizes the inguinal fat pad as a barrier to diminish the effects of the bed on the skin component of the groin flap. The flap in this model was designed obliquely over the groin fat pad which is located at the caudal aspect of the groin flap extending caudally to cranially and laterally.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








