Source
Date
Definition
Oxford English Dictionary
1643
Substance “that has the power of softening or relaxing the living animal textures”
Edwards (1940)
1940
“Since oils, fats and glycerin when applied to the skin tend to soften the epidermis they are termed emollients…”
“An adhesive coat is produced which prevents the irritation of drying, and the access of bacterial, chemical and mechanical irritants”
Blank (1957)
1957
Any material that tends to prevent or alleviate the symptoms and signs of dry skin
Idson (1982)
1982
Emollients – substances lubricating and/or occluding the skin with water-insoluble material (Moisturizers – substances actively increasing the water content of the skin)
Wilkinson and Moore (1982)
1982
Emolliency is only associated with imparting smoothness and general sense of well-being to the skin, as determined by touch
Wehr and Krochmal (1987)
1987
Emollients – systems that smooth the roughened surface of the SC, but do not occlude the skin. No effect on TEWL after application
Loden (1992)
1992
Similar action to moisturizers
Dederen et al. (2012)
2012
Emollients – oily ingredients used for skin care formulations
Other definitions of emollients have included the preparations themselves (Ray and Blank 1940; Harry 1941), and ointments designed for deeper skin penetration (Wild 1911). Confusion has arisen, in part, from the early emphasis on the emollient lipid film interactions with skin lipids and scales and the more mechanistic approach advocated by Blank, in 1957, which emphasized the skin hydration associated with emolliency. He defined an emollient as “any externally applied material that tends to prevent or counteract the symptoms and signs of dryness of the skin” (Blank 1957). An occlusive dressing is also often used to increase skin moisturization.
Figure 5.1 summarizes our current view of the effects of humectants, semipermeable or impervious occlusive films, semipolar emollients, and hydrocarbon emollients on stratum corneum (SC) roughness, hydration, and transepidermal water loss (TEWL). It is evident from this figure that emollients differ in their actions on normal skin, compared with other skin treatments such as application of humectants and occlusive dressings. As shown in Fig. 5.1, emollients affect both the transepidermal water loss and the roughness of skin surface through the oily film that they create. The lipid surface film on the stratum corneum and its resulting lubrication of the surface gives a feel of suppleness. The reduction in transepidermal water loss (TEWL) promotes skin hydration. The application of a semipolar emollient like vegetable oil is likely to reduce TEWL to promote skin hydration to a lesser effect than the more occlusive hydrocarbon emollient.
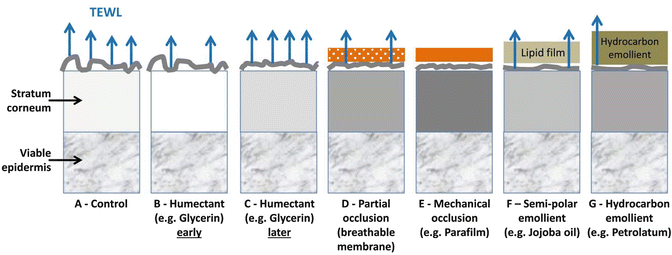

Fig. 5.1
The effect of various products on the stratum corneum, ranked in order of increasing lubrication of the stratum corneum surface after several hours of application (except B) for: (a). Control; (b). Humectant, early times only; (c). Humectant, later times; (d). Partial mechanical occlusion with a breathable membrane; (e). Mechanical occlusion, for example, with a plastic covering; (f). Semipolar emollient; (g). Hydrocarbon emollient. The figure also shows (i) the effect of products on stratum corneum hydration (the darker the box, the higher the skin hydration) and (ii) the transepidermal water loss (TEWL), with the number of arrows indicating the magnitude of the TEWL
We now describe the emollients used in practice, their potential effects on percutaneous absorption, and some practical examples of products containing emollients. In recognizing that the stratum corneum is the main barrier for both dermal and transdermal absorption, we have focused this chapter on the effects of emolliency on skin function and the skin penetration of the active.
5.2 Current Emollients, Their Modes of Action, and Their Use in Practice
5.2.1 Sebum
Sebum is the natural emollient of skin. It is produced from the sebaceous glands adjacent to the hair follicle and consists predominantly of squalene, wax esters, triglycerides, cholesterol esters, and possibly free cholesterol (Stewart 1992). The sebum provides a pliable film on the skin surface that lubricates the skin, inhibits percutaneous absorption of unwanted substances, and impairs TEWL, leading to increased skin hydration (Stoughton 1959). As well as providing lubrication and hydration of the skin, the sebum can also provide immunological and antimicrobial protection through its surfactant proteins and peptides, especially when their expression in human skin is upregulated (Mo et al. 2007). In addition, the sebum can also act as a buffer, impairing adverse irritation from acidic or basic compounds.
Regular washing of the skin, however, can remove the sebum and result in greater skin roughness and reduce stratum corneum hydration. Low sebum levels have been regarded as a contributing factor in the development of dry skin (Clarys and Barel 1995). Emollients are widely used to provide the desired lubrication and skin hydration that is normally supported by the sebum. In addition, a number of common skin care ingredients, including mineral oil, white petrolatum, and isopropyl myristate, have been shown to enhance sebocyte counts, and hence, potentially, sebum production, in a hairless mouse model (Lesnik et al. 1992).
Sebum has been shown to contribute to stratum corneum hydration by a glycerol-dependent mechanism. Based on the identification of glycerol as the putative product of triglyceride hydrolysis in sebaceous glands, Fluhr et al (2003) treated asebia mice, showing profound sebaceous gland hypoplasia, with glycerol, and were able to restore normal stratum corneum hydration. Urea, another commonly used humectant, had no effect.
5.2.2 Emollient Classes and Properties
Members of the different classes of emollients have different physicochemical properties that result in a range of functional and sensorial effects when left as a lipid film after being applied to the skin in a cosmetic or dermatological product. Traditionally, emollients have been regarded as having a number of common properties: (i) fat solubility, (ii) the ability to soften the skin, and (iii) an oily feel. However, they can differ quite markedly in their physicochemical properties. Some emollients are partially soluble in water (e.g., PEG-150 distearate, PEG/PPG-8/3 laurate) and are used not only for skin but also for hair (e.g., PEG-7 glyceryl cocoate, PPG-3 benzyl ether myristate). Others may feel dry to the touch (e.g., oleyl alcohol, C12–15 alkyl benzoate, phenethyl benzoate, cyclomethicone, and isononyl isononanoate). In general, the lipophilicities of the emollients in (Table 5.2) are such that those containing hydrogen bonding groups, such as ethers, esters, vegetable oils, and lanolin are more polar than those without these groups, for example, hydrocarbons. Today’s emollients are used to meet many different functional needs and to support multiple “claims,” and hence, a formulator has to select appropriate emollients to meet not only the consumer and regulatory needs but also to cater for whether the final product is designed for a cosmetic or a therapeutic application.
Table 5.2
Common emollients used in topical formulations
Chemical class | INCI name | CAS number | Physical form at 25°C |
|---|---|---|---|
Esters | Isopropyl palmitate | 142-91-6 | Liquid |
Isopropyl myristate | 110-27-0 | Liquid | |
Ethylhexyl palmitate | 29806-73-3 | Liquid | |
Octyl stearate | 109-36-4 | Liquid | |
Cetyl palmitate | 540-10-3 | Solid | |
Cetyl lactate | 35274-05-6 | Semi-solid | |
Myristyl lactate | 1323-03-1 | Semi-solid | |
C12–15 alkyl benzoate | 68411-27-8 | Liquid | |
Ethylhexyl isononanoate | 71566-49-9 | Liquid | |
Isononyl isononanoate | 59219-71-5 | Liquid | |
Cetyl isononanoate | 84878-33-1 | Liquid | |
Decyl oleate | 3687-46-5 | Liquid | |
Diisopropyl adipate | 6938-94-9 | Liquid | |
Diisobutyl adipate | 141-04-8 | Liquid | |
Glyceryl stearate | 123-94-4 | Solid | |
Propylene glycol stearate | 1323-39-3 | Solid | |
Glycol stearate | 31566-31-1 | Solid | |
Glycol distearate | 627-83-8 | Solid | |
Ethers | Dicaprylyl ether | 629-82-3 | Liquid |
PPG-15 stearyl ether | 25231-21-4 | Liquid | |
PEG-7 glyceryl cocoate | 68201-46-7 | Liquid | |
Triglycerides | Capric/caprylic triglycerides | 65381-09-01 | Liquid |
Fatty alcohols | Cetyl alcohol | 36653-82-4 | Solid |
Cetearyl alcohol | 8005-44-5 | Solid | |
Stearyl alcohol | 112-92-5 | Solid | |
Oleyl alcohol | 143-28-2 | Liquid | |
Octyldodecanol | 34513-50-3 | Liquid | |
Fatty acids | Oleic acid | 112-80-1 | Liquid |
Linoleic acid | 60-33-3 | Liquid | |
Hydrocarbons | Liquid paraffin | 8012-95-1/8042-47-5 | Liquid |
Petrolatum | 8009-03-8 | Solid | |
C9–14 isoparaffin | 246538-73-8 | Liquid | |
Polyisobutene | 9003-27-4 | Liquid | |
Isohexadecane | 93685-80-4 | Liquid | |
Vegetal butters | Butyrospermum parkii butter (Shea butter) | 194043-92-0 | Semi-solid |
Theobroma cacao seed butter (cocoa butter) | 84649-99-0 | Semi-solid | |
Mangífera indica seed butter (mango butter) | 90063-86-8 | Semi-solid | |
Vegetal oils | Prunus Amygdalus Dulcis seed oil (sweet almond oil) | 8007-69-0 | Liquid |
Vitis vinífera seed oil (grape seed oil) | 8024-22-4 | Liquid | |
Simmondsia chinensis seed oil (Jojoba oil) | 90045-98-0 | Liquid | |
Triticum vulgare germ oil (wheat germ oil) | 68917-73-7 | Liquid | |
Sesamum indicum oil (sesame oil) | 8008-74-0 | Liquid | |
Esterols | Lanolin | 8006-54-0 | Semi-solid |
Silicones | Cyclopentasiloxane | 541-02-6 | Liquid |
Dimethicone | 9006-65-9 | Liquid | |
Dimethiconol | 31692-79-2/70131-67-8 | Liquid |
5.2.3 Effect of Emollients on Skin Lubrication
The choice of an emollient is often based on the tactile properties of these substances on the skin surface and is often of higher importance in cosmetology than in the formulation of topical therapeutic drugs, where sensory properties are not necessarily the main priority (Dederen et al. 2012). A plethora of imaginative terms may be used to describe these subjective properties. Words such as “tacky,” “oily,” “dry-velvety,” or “waxy” are readily understood, whereas other more esoteric terms such as “scroopy” (the textile chemist’s description of the rough, soft-draggy feel of raw silk) are less obvious (Goldemberg and De La Rosa 1971). All these terms are used in an attempt to describe the sensory responses caused by the lubricating actions of emollients on the skin. A special issue of the Journal of Investigative Dermatology was devoted to the effects of emollients on the mechanical properties of the skin in 1977, with articles on the viscoelastic (Christensen et al. 1977) and frictional (Highley et al. 1977) properties of human skin, as well as measurement of skin hydration (Campbell et al. 1977), among others.
To the formulator, the tactile sensory properties of the neat oils are the first consideration in choosing an emollient for a cosmetic product (Goldemberg and De La Rosa 1971; Zeidler 1992). The key property of the emollient that this is reflecting is its ability to lubricate and reduce any friction between the skin surface and its environment (skin with skin, clothing with skin, etc.), as this reduces possible discomfort, irritation, and pain (Dederen et al. 2012). The lubrication intensity of the emollient on the skin can be partly explained by the properties of the emollient itself; the residual film thickness, by dynamic spreadability and the viscosity. However, the skin is not a rigid, inert surface, and emollients can directly or indirectly modify its mechanical properties. This must also contribute to the overall sensory response (Dederen et al. 2012). This important property of emollients is defined by their ability to disperse more or less quickly on the skin surface by forming a film. This can be assessed quantitatively using a parameter known as the spreading value. A common technique for determining the spreading values has been described by Zeidler (1985). Spreading values, in units of mm2/10 min, are determined by applying 20 μl of an emollient to the center of an ashless, medium-to-fast filter paper at 25 °C and measuring the area covered by the applied material in 10 min. Examples of spreading values for some of the most widely used group of emollient for skin lubrication, the esters, are shown in Table 5.3. Esters are useful to formulators because of their versatility and the unique properties they can give to the final product, influenced by the chemical properties, including chain length, of their constituent fatty acids and alcohols. As can be seen, changes in the constituent chain lengths can alter the skin-surface spreading characteristics of ester emollients. For example, isopropyl myristate and palmitate, with short-chain alcohol components and the shortest acid chain lengths (C14 and C16, respectively) in this table, have the highest spreading values. The C16 palmitate is greasier than the C14 myristate, but the spreading values are similar. Ethylhexyl stearate and decyl oleate, with longer chain components, have medium spreading values, whereas the longer chain alcohol (C12–C15), alkyl benzoates, are low spreading esters.
Table 5.3
Spreading values for selected ester emollients
High spreading values | >850 mm2/10 min | For example, isopropyl myristate and isopropyl palmitate |
Medium spreading values | 501–850 mm2/10 min | For example, ethylhexyl stearate and decyl oleate |
Low spreading values | 0–500 mm2/10 min | For example, C12–15 alkyl benzoate |
Many attempts have been made to achieve a measure of sensory softness of the skin. In 2013, Nakatani suggested that conventional methods for measuring the mechanical properties of the skin, such as the elongation in response to suction, elastic responses to ballistic impact, and rheological responses to torsional stress, were restricted to measuring the properties of the whole skin and were unable to look at different skin layers separately. They developed a novel piezoelectric tactile sensor system that could simultaneously measure the mechanical properties of the whole skin and its superficial layer. Such a technique has obvious advantages to the cosmetic industry, but can also be applied clinically to the quantitative evaluation of skin disorders such as atopic dermatitis (Nakatani et al. 2013).
5.2.4 Effect of Emollients on Skin TEWL and Skin Hydration
The residual lipid film on the stratum corneum surface after the application of products containing emollients will limit the evaporation of water from the skin surface and therefore cause an increase in skin hydration. Accordingly, emollients have been described as indirect skin moisturizers (Dederen et al. 2012). In general, the presence of hydrogen bonds in emollients also facilitates the transport of water through the lipid films, so that for lipid films of similar thickness and viscosity, the semipolar emollients will be more permeable to water than the hydrocarbon emollients, resulting in a lower occlusive state than that induced by the hydrocarbon emollients. However, these findings can differ significantly, depending on the nature of the emollient. Patzelt et al (2012) recently showed that vegetable oils (except Jojoba oil) led to a similar occlusion of the human skin surface in vivo as paraffin oil, but the semisolid, petrolatum, was the most effective occlusive. The occlusive effects of an emollient then result in a reduced transepidermal water loss (TEWL) and, in turn, an increase in the hydration of the stratum corneum relative to normal moisture conditions. By occluding the skin and providing an additional barrier to water loss, skin hydration can be increased by up to 50 % (Hafeez and Maibach 2013a). This increase in hydration as an effect of occlusion has also been seen with physical occlusives like wound dressings and bandages (Voegeli et al. 2009, 2011). Increasing the thickness of the lipid film and/or increasing the viscosity of the lipid film will reduce the TEWL and increase stratum corneum hydration, so that a wax will have a low TEWL and higher SC hydration than an oil.
The presence of water in a formulation can add to the moisturizing properties of that formulation on the skin, but generally for only a short time. Indeed, the moisturizing effect of topically applied water is often lost after 10–20 min of application (Batt and Fairhurst 1986; Paepe and Rogiers 2009). Blank showed that the main effect associated with skin moisturization was an increase in its softness and pliability (Blank 1952). The moisturizing effect of water can be prolonged when an emollient is present in the moisturizing formulation. The presence of a humectant, such as glycerol, in the aqueous phase, as well as the emollient will increase the moisturization of the skin. Indeed, Batt et al. showed that the enhanced moisturizing effect of glycerol by different emollients and oils was present even 12 h after application (Batt et al. 1988).
Nonphysiological occlusive moisturizers such as petrolatum remain on the skin surface without being incorporated into the deeper skin layers. While they may provide some benefit by improving skin hydration, they are not effective in directly treating the disordered lipid states in such diseases. For example, petrolatum treatment had no effect on the abnormal lipid organization associated with barrier defects in patients with atopic dermatitis or laminar ichthyosis (Pilgram et al. 2001). On the other hand, some moisturizers do act by penetrating the intercellular lipid regions. A novel mechanism known as “internal occlusion” (Wiechers et al. 2009) has been described, where moisturizers such as isostearyl isostearate and isopropyl isostearate cause improved skin hydration and barrier function by stabilizing the SC lipid organization in the more tightly packed orthorhombic phase (Caussin et al. 2007).
A different approach to the emollient treatment of skin diseases such as atopic dermatitis, relying on the use of emollient treatments containing ceramide-dominant physiological mixtures of the three key lipids, cholesterol, free fatty acids, and ceramides at the appropriate physiological pH, has been pioneered by Elias (Chamlin et al. 2002; Elias 2010). The mechanism leading to skin barrier enhancement is believed to involve more than a simple augmentation of intercellular lipid populations and structure. On passing through the SC, the applied lipids migrate to the nucleated cell regions, to be taken up by keratinocytes and then trafficked to lamellar bodies, where they are mixed with endogenous epidermal lipids. The augmented lipid mixture is then secreted into the SC intercellular spaces (Mao-Qiang et al. 1995; Chamlin et al. 2002) to enhance skin barrier function and normalize skin hydration. According to Elias, the effectiveness of any such treatment depends primarily on understanding the mechanism responsible for a particular skin barrier defect, in order to judge whether lipid replacement is appropriate for that condition (Chamlin et al. 2002). An alternative approach to address imbalance in the SC proteolytic cascade leading to dry skin is to use serine protease inhibitors to treat mild-to-severe barrier abnormalities (Voegeli et al. 2009; Rawlings and Voegeli 2013).
5.2.5 Emollient Substantivity
This is defined here as a measure of the retention of an emollient in and persistence of its effect on the skin after exposure to water, perspiration, and resistance to being rubbed off. Another definition of substantivity is: “the property of continuing therapeutic action despite removal of the vehicle, such as the action of certain shampoos” (Mosby 2009). (MediLexicon 2013), referring to Stedman’s Medical Dictionary (Stedman’s 2011), suggests substantivity is a “term comprising the adherent qualities of a sunscreen and its ability to be retained after the skin is exposed to water and perspiration. Persistence of effect of a topically applied drug or cosmetic, determined by the degree of physical and chemical bonding to the surface; resistance to removal or inactivation by sweating, swimming, bathing, and friction, among other factors.” In general, emollient substantivity is poor for an aqueous gel but comparatively better for an O/W emulsion. Greater substantivity would be expected for a W/O emulsion and more particularly for an ointment. It has been suggested that silicone ingredients have a higher substantivity than the more common emollients, as silicone chains are entangled (Sene 2003).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree