Chapter 22 The immunological response and strategies for intervention
![]() Access the complete reference list online at http://www.expertconsult.com
Access the complete reference list online at http://www.expertconsult.com
Introduction
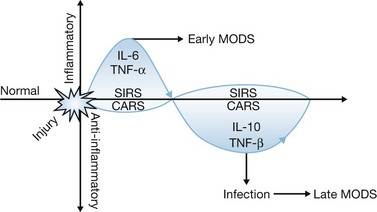
Despite improvements in the management of burn injury1,2 and the development of broad-spectrum antibiotics, infections still remain a major cause of morbidity and mortality in burn patients.3,4 Many infections are associated with organisms considered to be opportunistic in nature, which supports the concept that antimicrobial function may be impaired in these patients. One paradigm of burn-induced immune dysfunction asserts that the initial burn injury induces a severe inflammatory response characterized by the systemic release of proinflammatory cytokines, chemokines and acute-phase reactants that cause leukocyte and endothelial cell activation (Fig. 22.1). This initial inflammatory response has been termed the systemic inflammatory response syndrome (SIRS) and causes a variety of hemodynamic, immunologic and metabolic responses that can lead to significant tissue injury and organ dysfunction. However, if adequately resuscitated, most burn victims survive the initial injury and enter a phase characterized by varying degrees of immune dysfunction. This state of immunologic dysfunction, along with the loss of the skin barrier and the use of invasive instrumentation such as central venous catheters, peritoneal dialysis catheters and endotracheal tubes, places the burned host at increased risk of infection. Some practitioners have proposed that post-burn immunologic dysfunction is caused by an anti-inflammatory response, termed the counter anti-inflammatory response syndrome (CARS), which is initiated by the host in an effort to minimize inflammation-induced tissue injury during the acute post-burn period. CARS is characterized by a relative preponderance of anti-inflammatory cytokine production, leukocyte apoptosis, regulatory lymphocyte generation and a shift in adaptive immunity from a Th1 to a Th2 phenotype. Accumulation of inflammatory insults during the post-burn period may potentiate injury-induced immunosuppression. Therefore, there is keen interest in developing immunomodulatory strategies aimed at normalization, or enhancement, of antimicrobial function. Interventions that can improve the host response to infection are also becoming increasingly attractive because of the emergence of antibiotic-resistant microorganisms as pathogens in burn patients.5,6 However, few effective immunomodulatory approaches have been developed for clinical application in burn patients. This may be because immune dysfunction parallels failure of other organ systems and may be a reflection of multiorgan system dysfunction. Leukocytes in that environment appear to be ineffective in responding to infection, and may also be refractory to immunomodulatory interventions. Therefore, the early initiation of immunomodulation may be most advantageous, although further research is needed to determine whether immunomodulation will be effective in burn patients and which approaches have the most potential for improving overall outcome.
The depth and size of the burn wound are well-known risk factors for infection and sepsis.4,7 The overall incidence of bacteremia in burn patients is approximately 19%, but bacteremia rarely occurs in patients with <40% TBSA burns.8 Concomitant inhalation injury is also associated with an increased risk of infection, particularly pneumonia. The predisposition to pneumonia seen in patients with inhalation injury is likely due to impairment of mucociliary clearance, alveolar macrophage dysfunction, and airway obstruction with casts and debris in conjunction with a high incidence of tracheal intubation and mechanical ventilation.9
Some investigators have reported a gender effect on post-burn mortality, with females having a lower chance of survival.10 However, a study of children did not show a relationship between gender and the incidence of sepsis.11 Nevertheless, age appears to be a predisposing factor for the development of septicemia after burn injury. Not surprisingly, young children and elderly patients have the highest incidence of sepsis.12
Relative to other catheters, central venous catheters are particularly prone to bacterial contamination. Approximately 25% of central venous catheters become colonized with bacteria. Lesseva et al. reported an incidence of catheter-related bacteremia of 6.6%, accounting for approximately 20% of sepsis in burn patients.13 Peripheral catheters are much less likely to be associated with infection because of their shorter duration of use. The risk of catheter-related infection is greatly increased if devices are inserted through infected or contaminated skin, such as burn wounds. Femoral vein catheters are more likely to become infected than subclavian or internal jugular vein catheters, and catheter replacement via guide wire exchange has a higher infection rate than catheterization at a separate site. One of the most common causes of Gram-negative sepsis in hospitalized patients is urinary tract infection, especially in the presence of Foley catheters. Nearly all patients with large TBSA burns require bladder catheterization. The incidence of urinary tract infection in burn patients ranges from 10% to approximately 18%.3 The incidence of respiratory infections in burn patients ranges from 20% to 40%.14 Nosocomial pneumonia is frequently associated with prolonged intubation and mechanical ventilation.15 Gram-negative bacteria such as Pseudomonas aeruginosa are commonly isolated, but Gram-positive bacteria and yeasts such as Candida spp. should also be considered.
Medications administered to burn patients may in some instances also be a contributing factor to post-burn immunosuppression and increased susceptibility to infection. Despite the need for aggressive administration of systemic antibiotics, there is an increasing emergence of antibiotic-resistant bacteria in burn patients which has significantly affected infection control efforts.16 In addition, some clinical reports indicate that systemic antibiotic administration may be a predisposing factor for the development of fungal infections.17,18 Recent experimental work suggests that administration of opiate analgesics may contribute to post-burn immunosuppression by adversely affecting T-cell proliferation and altering the predominant T-helper phenotype.19 Transfusion of blood products has also been reported to predispose burn victims and other critically ill patients to nosocomial infections.20 Although the exact mechanism of immunosuppression is unknown, blood transfusions have been associated with a decrease in the T-helper–lymphocyte ratio, as well as adverse effects on natural killer cell activity and antigen presentation.21,22 Finally, victims of large TBSA burns usually require numerous operative procedures for debridement and grafting of burn wounds. Intravenous and inhalation anesthetics have demonstrated variable and inconsistent effects on neutrophil function, including alterations in chemotaxis, endothelial adherence and transmigration, phagocytosis, and respiratory burst, and could contribute to post-injury immunosuppression.23
Innate immune function after burn injury
After a large burn injury with extensive tissue necrosis, the innate immune system is activated both locally and systemically. Subsequent dysfunction of innate immune function may lead to increased susceptibility to infections. Study of immune function in burn patients is limited by feasible tissue access and generally consists of measuring circulating soluble factors and immune cells. Not surprisingly, this provides only a limited view of the entire immune response, as suggested by animal studies in which alternate tissues such as spleen and liver are available.24–27 This review will highlight observational data from clinical studies and try to use results from experimental research to provide some context for these observations.
Physical barriers
A mechanical barrier against invasion of microbial pathogens is provided by the epithelium of skin and mucosal tissue. This protective barrier is lost after destruction by full-thickness burn injury. Furthermore, the presence of necrotic tissue provides a favorable environment for microbial growth. Cellular damage may also cause the release of molecules that predispose the host to bacterial infections. Syndecans are heparin sulfate proteoglycans that regulate innate immune responses, and their release after cellular damage activates tissue repair processes, including vascular permeability, angiogenesis, wound repair, and modulation of chemokine activity.28 In general, syndecans have been shown to attenuate inflammatory responses and protect tissues from inflammatory injury. However, recent studies in burned mice suggest that syndecan shedding may potentiate systemic dissemination of P. aeruginosa from burn wound infections, an effect that may be attenuated by the application of protamine.29,30
Besides the loss of epidermal integrity in burned skin, barrier function may also be compromised at other remote anatomic locations. Epithelial barrier function in the gastrointestinal tract can be compromised after burn injury, possibly owing to decreased mesenteric blood flow in the early post-burn period.31 This may lead to systemic translocation of bacterial toxins or enteric bacteria. Increased intestinal permeability after burn injury has been associated with systemic infection in a number of studies, and forms the basis for the therapeutic strategy of selective decontamination of the digestive tract as well as interventions aimed at improving gut barrier function, such as early enteral feeding.32 In the respiratory tract, defense mechanisms include mucus secretion and ciliary movement, which serve to trap microbes and sweep them into the upper airways and oropharynx, where expulsion by coughing may occur. These defenses can be diminished after inhalation injury or compromised by endotracheal intubation, resulting in microbial proliferation and expansion into the distal branches of the respiratory tract, causing bronchitis or pneumonia.33,34 Smoke inhalation also facilitates the development of bronchial casts composed of neutrophils, sloughed respiratory mucosa and fibrinous products, which impair microbial clearance mechanisms and aid bacterial growth.35 Pneumonia is one of the most common infections encountered in burn patients and is associated with a higher risk of mortality.36
Epithelial cells contribute further protection by manufacturing antimicrobial peptides, including defensins, lysozymes, and cathelicidins. Defensins exert antimicrobial activity against a broad range of bacteria and fungi and appear to function through disruption of the bacterial cell wall.37 Although epithelial cells display some constitutive production of defensins, production is increased after microbial activation of Toll-like receptors (TLRs) on the epithelial cells.38 However, burn injury has been associated with decreased defensin mRNA expression, and it is not clear that microbial stimulation would invoke an appropriate level of activation in affected areas.39
Phagocytes and Toll-like receptors
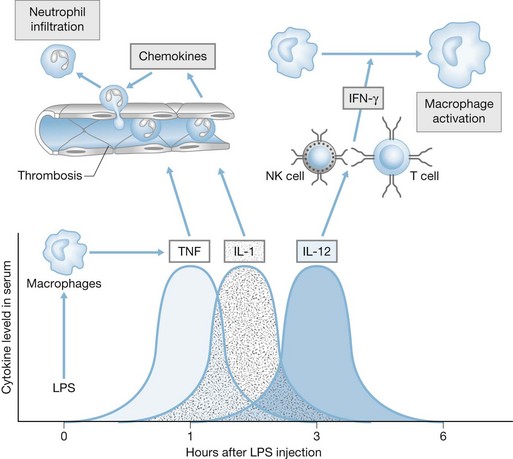
Beyond the physical barriers, the key cellular components of the innate immune response are the professional phagocytes, including macrophages, dendritic cells and neutrophils, which are not only important in removing microbes and debris from sites of inflammation but also are key regulators of the innate immune response (Fig. 22.2). Macrophages are capable of engulfing and killing microbes, but probably function most importantly as supervisors of the immune response to infection. They elaborate chemotactic factors for the recruitment of other cells, particularly neutrophils, to the site of infection. Along with dendritic cells, macrophages also function to initiate the adaptive immune response by presenting bacterial antigens to CD4+ T cells. Neutrophils are effective at killing microbes through an arsenal of specialized antimicrobial functions (discussed below). Because the innate immune system must react to potential pathogens before they proliferate and disseminate, detection is dependent on the recognition of microbial molecules that are consistently found among a vast assortment of microorganisms.
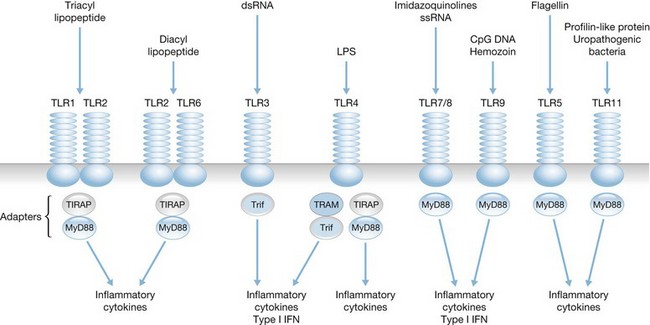
Toll-like receptors, found on mononuclear phagocytes, provide a primitive, non-specific mechanism of pathogen recognition based on the detection and binding of conserved microbial products rather than recognition of specific microbial antigens.40 Human leukocytes express at least 10 TLRs, which recognize microbial ligands such as lipopolysaccharides, lipoproteins, lipotechoic acid, flagellin, unmethylated bacterial CpG DNA, and viral RNA, as well as endogenous ligands such as heat shock proteins, HMGB-1 and mitochondrial DNA (Fig. 22.3). TLR ligation results in activation of specific signaling pathways that cause nuclear translocation of transcription factors such as NF-κB, AP-1 and IRF-3, which induce transcription of genes encoding proinflammatory gene products such as cytokines, chemokines and nitric oxide synthase.41,42
The importance of TLRs for recognition and clearance of microbes has been demonstrated in studies using mice that are genetically deficient of specific TLRs. TLR-deficient mice are highly susceptible to microbial infections owing to the inability to effectively respond to infection and clear invading organisms.43–45 Furthermore, analysis of a cohort of humans with congenital loss of MyD88, an important signaling intermediate required by most TLRs, showed a high incidence of infections with Pseudomonas spp, streptococci and staphylococci, which are common opportunistic pathogens among burn patients.46 It is not known whether alterations in TLR expression or function directly contribute to impaired innate antimicrobial functions in burn patients. However, Barber and colleagues have reported an association between TLR polymorphisms and the development of sepsis in burn patients.47 In addition, several studies indicate that TLR activation contributes to the systemic inflammatory response and associated alterations caused by thermal injury, such as altered vascular and gut permeability, myocardial dysfunction and microvascular inflammation.48–50 Macrophages seem primed for innate immune activity after burn injury through increased TLR4 expression and increased reactivity to LPS challenge, which may predispose burn victims to further inflammatory injury.25,51
Neutrophil microbicidal function
Large-scale neutrophil infiltration is characteristically seen in burn wounds. Although this is probably an advantageous response to protect against potential pathogens, it may also be associated with some undesirable biologic effects. Oxidants and hydrolytic enzymes released from activated neutrophils can contribute to proteolysis within the wound and aid in the spread and dissemination of bacteria. Abnormal neutrophil function has been associated with bacteremia in patients with burns and other major injuries.52 Neutrophil microbicidal mechanisms are generally classified as either oxygen dependent or oxygen independent. Oxygen-independent microbicidal activity of neutrophils involves several peptides and proteins stored within the primary (azurophilic) granules, including lysozyme, bactericidal/permeability-increasing protein, β-defensins, and cathelicidin. In the oxygen-dependent mechanism, NADPH oxidase in the phagosome of neutrophils (and macrophages) converts molecular oxygen into superoxide anion (O2–), and a series of reactions creates further oxidant products with microbicidal activity, including H2O2, OCl− (hypochlorite) and NH3Cl and RNH2Cl (chloramines). A reduction in neutrophil-mediated oxygen-dependent bacterial killing has been reported in several studies of burn patients.53,54 Poor tissue perfusion and low oxygen tension in wound sites are additional factors likely to contribute to impaired oxidative killing. Chemotactic gradients are provided by chemoattractants such as the complement protein C5a, endothelium-derived IL-8, and leukotrienes. Chemoattractants and other stimuli also induce increased expression of surface phagocytic receptors. Bonding between phagocyte receptors and opsonins on microbial surfaces activates cytoskeletal contractile elements, resulting in invagination of the cell membrane and extension of pseudopods around the microbe. Clinical studies of neutrophils from burn patients demonstrate a reduction in neutrophil adhesion molecule expression and phagocytic activity.53,55 Theoretically, these alterations could lead to decreased innate antimicrobial activity and increased susceptibility to infection. However, a specific cause and effect relationship between altered neutrophil function and the development of infections in burn patients has not been firmly established.
Innate lymphocytes – natural killer (NK), natural killer T (NKT), and γδ T cells
Natural killer (NK) cells are lymphoid cells that do not express T-cell receptors (TCR) or immunoglobulins on the cell membrane but rapidly respond to infections through activating specific chemokine receptors and other activating innate receptors. NKT cells express both NK cell markers and restricted T-cell receptors (TCRs) that respond primarily to lipid antigens presented by the antigen-presenting molecule CD1d. γδ T cells comprise about 10% of all T cells and play important immunoregulatory roles in skin, gut, respiratory tract and other mucosal sites. All three innate lymphocytes are able to induce dendritic cell (DC) maturation through a combination of cell contact-dependent mechanisms and cytokine signaling. In turn, matured DCs can stimulate NK, NKT, and γδ T cells to sustain the innate immune response during the development of adaptive immunity. γδ T cells, which are in found in high numbers in intestine, may play a role in the recruitment of neutrophils to that site after burn injury.56 Some studies indicate that γδ T cells facilitate neutrophil-independent and neutrophil-mediated organ injury after burn injury.57,58 NK cells are best known for their role in containing viral infections prior to the adaptive immune response, as well as assisting in the control of malignant tumors. Activated NK cells are an important source of IFNγ, which promotes the development of specific protective immune responses but may also contribute to the systemic inflammatory response after severe burn injury.59 NK cells kill infected cells through the release of perforin and granzymes and through the binding of the death receptors Fas and TRAIL-R on target cells. NK cells also interact with other cells of the immune system, including dendritic cells, by providing signals for maturation or apoptosis. Studies of patients after burn injury have revealed a decline in NK-cell activity over time.60 The decline in NK cell activity is most apparent more than 1 week after the occurrence of the burn injury and corresponds with the period during which sepsis is more likely to be identified.60
Complement
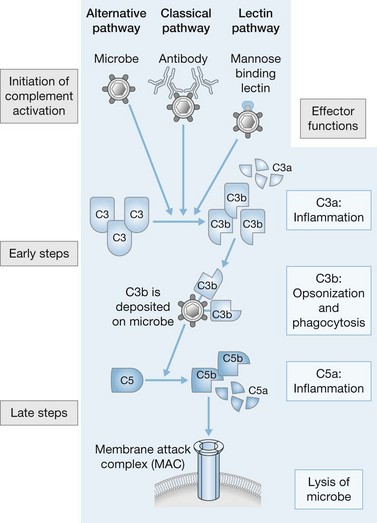
As well as Toll receptors, the innate immune system has additional mechanisms for recognizing and neutralizing microbial pathogens, including the complement system, scavenger receptors, and specialized receptors on NK cells. Activation of the complement system results in the production of a cascade of proteins that function to opsonize microbes and recruit phagocytes. Three distinct pathways constitute the complement system: the classic pathway, the mannan-binding lectin pathway, and the alternative pathway (Fig. 22.4). Binding of IgG or IgM to antigens on microbial surfaces activates the classic pathway. The remaining two pathways can be activated in the absence of specific antibodies. The mannan-binding lectin pathway is similar to the classic pathway but is initiated after mannan-binding lectin protein binds to mannose-containing carbohydrates on microbial surfaces. The alternative pathway is dependent on low constitutive expression of complement activity which is amplified and potentiated when circulating complement components bind to microbial membranes. All three of the activation pathways act on microbial surfaces to assemble a convertase that cleaves C3 to form C3b, which either binds to the surface as an opsonin or helps activate C5 and the remainder of the complement cascade. Opsonization facilitates the removal of microbes by macrophages and neutrophils. C3a and C5a are potent chemoattractants for monocytes and neutrophils. Cleavage of C5 also leads to assembly of the membrane attack complex, which causes disruption of the microbial membrane. After burn injury, neutrophil surface expression of receptors for complement is increased, but the complement cascade itself is diminished and may serve as a prognostic indicator.61,62 In a clinical study of severely burned patients (>60% TBSA, third-degree) serum concentrations of complement proteins were measured sequentially. All patients had decreased concentrations of C3 initially; however, serum concentrations of C3 rebounded fully in surviving patients while remaining depressed in non-survivors.63 Diminished complement, in combination with decreased concentrations of fibronectin and serum immunoglobulins, contributed to the depression of opsonic activity in both serum and blister fluid from burn patients, and may predispose burn patients to wound infections.64
Cytokines and chemokines
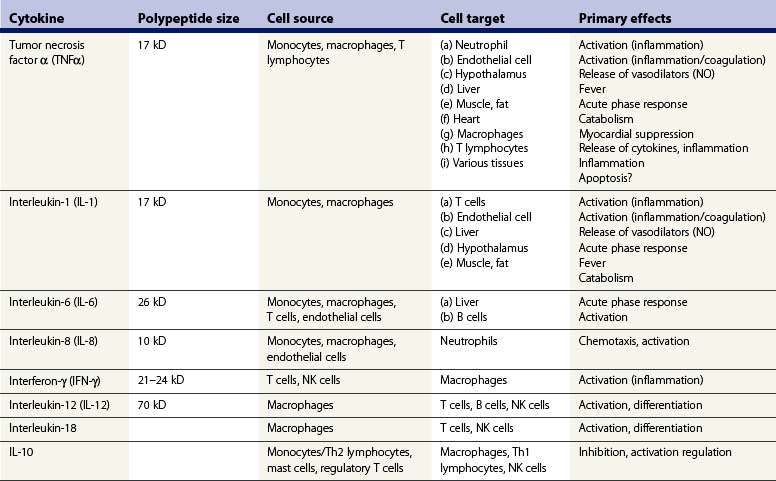
Cytokines are a heterogeneous group of small polypeptide-soluble mediators that are important for the regulation of antimicrobial immunity. In conjunction with hormones and neurotransmitters, cytokines regulate tissue repair and immune responses. Most cells of the immune system either release cytokines and/or respond to cytokines through specific receptors. The cytokine response evoked by microorganisms controls both innate and adaptive immune responses, including inflammation, defense against viruses, leukocyte recruitment and proliferation of specific T- and B-cell clones, while regulating the function and interaction of a variety of immune cells. A complete discussion of cytokine biology is beyond the scope of this chapter. It is likely that a broad array of cytokines regulate the pro- and anti-inflammatory changes that occur after burn injury. Some of the most recent reports on cytokine function in burn patients are reviewed below, and a brief list of cytokines that have been studied in burned populations is presented in Table 22.1.
The description of the cytokine response after burn injury has varied widely in several clinical studies. It is likely, based on accumulated evidence and results from well-controlled animal experiments, that burn injury by itself evokes only a modest increase in circulating cytokines. This limited cytokine response may be related, in part, to burn-induced expression of anti-inflammatory signaling pathways and factors such as suppression of cytokine signaling-3 (SOCS 3) within hours of burn injury.65 Nevertheless, concentrations of cytokines remain elevated in plasma for weeks after major burn injuries, and there appears to be a significant difference in cytokine production between children and adults.66 Cytokines may also be elevated in the circulation of post-burn patients because of stresses during treatment, including intubation and ventilation, surgery, transfusions, and exposure to microorganisms. In fact, there is evidence to suggest that the cytokine response to a secondary insult, such as infection, may be greater after burn injury than under basal conditions.67 Burn injury appears to ‘prime’ immune and inflammatory cells for an exaggerated response to endotoxin and other microbial products.68,69 Post-burn ‘hyperreactivity’ in terms of cytokine production has been well described in macrophages.70 Priming for an exaggerated response to LPS stimulation has also been described in alveolar macrophages harvested from bronchoalveolar lavage of smoke inhalation patients or from rabbits after experimental smoke inhalation injury.71 Interestingly, although surgery may potentially invoke an inflammatory response, burn wound excision has been associated with a reduction in circulating concentrations of endotoxin and cytokines, likely resulting from the removal of inflammatory stimuli such as necrotic tissue and bacteria.66,72
IL-6 is one of the most consistently elevated cytokines in the circulation of post-burn patients and experimental animals.67,73 A recent study indicates that elevated plasma IL-6 concentrations may correlate with mortality in patients with burn and smoke inhalation injuries.74 It is unclear what specific function IL-6 has in the burn-induced immune response, but a study showed improved cell-mediated immunity after blockade of IL-6 in burned mice.75 In another study, the proinflammatory response and mortality were attenuated in post-burn mice subjected to endotoxin when IL-6 was blocked.76 IL-6 has also been shown to contribute to myocardial dysfunction during the post-burn period. Horton and colleagues have described the contribution of IL-6 to burn-induced myocardial dysfunction in several experimental studies, an effect that is likely orchestrated by numerous proinflammatory mediators and initiated by activation of Toll-like receptors.77–79
Perhaps more important than the burn-induced cytokine response is the secondary cytokine response to potential pathogens in the post-burn period. In this regard, burn injury may be followed by a period of attenuated expression of some cytokines that are important in the immune response to microbial organisms, and increased expression of cytokines that may hinder the immune response. For example, expression of IFNγ and a number of IFNγ-inducing cytokines, including IL-2, IL-12, and IL-15, is reduced in response to a secondary immune stimulus after burn injury.80,81 IFNγ facilitates numerous beneficial effects on the immune response to infection, including induction of class II major histocompatibility complex (MHCII) proteins on antigen-presenting cells. Antigen-presenting cells such as macrophages and dendritic cells use the MHCII as a mechanism for presenting microbial antigens to CD4+ T cells at sites of infection. Decreased monocyte expression of the MHCII is a consistent finding in burn patients.82,83 IFNγ activity also contributes to phagocytosis and microbial killing through several mechanisms. In mice, IFNγ induces IgG2a, which opsonizes bacteria and promotes the expression of FcγR1 receptors on phagocytes. IFNγ also potentiates the availability of NO, hydrogen peroxide, and superoxide in phagocytic cells, and promotes expression of chemotactic factors and adhesion molecules for mononuclear cell recruitment to areas of infection. In experimental burn models, lack of IFNγ activity has been associated with diminished ability to clear bacteria.81
Impaired production of IL-12 in response to endotoxin has also been described in mice after burn injury.81 IL-12, an important product of dendritic cells and monocytes, serves to stimulate production of IFNγ by NK cells as well as playing a role in determining the class of T-helper lymphocyte response. IL-10 is a cytokine that has anti-inflammatory activity and may serve to attenuate the proinflammatory response and prevent excessive inflammation after burn injury. Increased and prolonged production of IL-10 may contribute to immunosuppression in the post-burn period. Immune cells from burn patients demonstrated an exaggerated IL-10 response to secondary stimulation with endotoxin or other microbial products, and this finding correlated with septic episodes. Experimental studies confirmed an increased IL-10 response to microbial stimuli in animals that had previously been subjected to thermal injury.81,84 Concentrations of IL-10 have been reported to reach a peak in the plasma of patients approximately 1 week after burn injury, which correlates with the time when septic episodes begin to occur with increased frequency.85 In another clinical study, serum concentrations of IL-10 appeared to be highest in non-surviving patients with sepsis.86 However, the functional role of increased IL-10 and decreased IFNγ production in facilitating post-injury immunosuppression is not completely clear. Murphey and colleagues87 reported that IL-10 neutralization and IFNγ administration did not improve survival or bacterial clearance in a model of post-injury immunosuppression.
Chemokines or chemotactic cytokines are a group of specialized polypeptides that play a complex role in the innate immune response by directing migration of immune and inflammatory cells. They are named and grouped based on the position of cysteine residues near the amino terminus, which determines the typical three-dimensional structure of these polypeptides (Table 22.2). The leukocytes and endothelial cells of most inflamed tissues can release a variety of chemokines that recruit specific sets of leukocytes to a site of infection, based on the nature of the inciting pathogen. Chemokines also recruit leukocytes to sites of tissue injury such as the burn wound. IL-8, a neutrophil-specific chemoattractant, is one of the most widely studied chemokines. IL-8 has been reported to be significantly elevated in the circulation of post-burn patients, and the highest concentrations are detected in non-survivors.88,89 Severe burn injury is often associated with a period of neutropenia, perhaps as a function of neutrophil activation and exit from the circulation. Besides the burn wound, the lung is a preferred site for neutrophil sequestration in burn patients, even in the absence of inhalation injury. Experimentally, neutrophils can appear in lung tissue within hours of burn injury, and this phenomenon can be attenuated by neutralization of complement C5a or of the chemokines keratinocyte-derived cytokine (KC) or macrophage inflammatory protein-2 (MIP2).90–92 MIP-2 is an IL-8 homolog in the mouse. Furthermore, Schmalsteig and colleagues have shown that IL-8-mediated neutrophil recruitment is a central factor contributing to pulmonary injury caused by burns and smoke inhalation.93,94 However, several studies have demonstrated diminished neutrophil chemotaxis in post-burn patients which may, in part, be due to a combination of suppressive circulating soluble factors and a reduced ability to upregulate the MAC-1 (CD11b/CD18) adherence receptors on the cell surface.95,96 A recent clinical study of burn patients demonstrated an association between the development of bacteremia and a decrease in neutrophil expression of CD11b, an adhesion molecule that functions in endothelial binding and migration from the circulation.97 Overall, it is likely that burn injury has significant effects on neutrophil function, which is dependent on the size of the burn, the time elapsed after burn injury, the specific tissue being analyzed, and the specific stimulus that is eliciting the neutrophil response.
Table 22.2 Classification of chemokines
| Chemokine type | Target cell |
|---|---|
| CXC chemokines | |
| CXCL8 (IL-8, mouse MIP-2) | Neutrophils |
| CXCL1 (GROα, mouse KC) | Neutrophils |
| CXCL2 (GROβ, mouse KC) | Neutrophils |
| CXCL3 (GROγ, mouse KC) | Neutrophils |
| CXCL5 (ENA-78) | Neutrophils |
| CXCL6 (GCP-2) | Neutrophils |
| CXCL4 (PF4) | Fibroblasts, stem cells |
| CXCL9 (Mig) | T and NK cells |
| CXCL10 (IP-10) | T and NK cells |
| CXCL11 (I-TAC) | T and NK cells |
| CXCL12 (SDF-1α/β) | T lymphocytes |
| CC chemokines | |